HIV-1 Nef promotes the localization of Gag to the cell membrane and facilitates viral cell-to-cell transfer
- PMID: 23899341
- PMCID: PMC3734038
- DOI: 10.1186/1742-4690-10-80
HIV-1 Nef promotes the localization of Gag to the cell membrane and facilitates viral cell-to-cell transfer
Abstract
Background: Newly synthesized HIV-1 particles assemble at the plasma membrane of infected cells, before being released as free virions or being transferred through direct cell-to-cell contacts to neighboring cells. Localization of HIV-1 Gag precursor at the cell membrane is necessary and sufficient to trigger viral assembly, whereas the GagPol precursor is additionally required to generate a fully matured virion. HIV-1 Nef is an accessory protein that optimizes viral replication through partly defined mechanisms. Whether Nef modulates Gag and/or GagPol localization and assembly at the membrane and facilitates viral cell-to-cell transfer has not been extensively characterized so far.
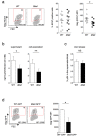
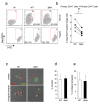
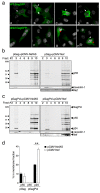
Results: We report that Nef increases the total amount of Gag proteins present in infected cells, and promotes Gag localization at the cell membrane. Moreover, the processing of p55 into p24 is improved in the presence of Nef. We also examined the effect of Nef during HIV-1 cell-to-cell transfer. We show that without Nef, viral transfer through direct contacts between infected cells and target cells is impaired. With a nef-deleted virus, the number of HIV-1 positive target cells after a short 2h co-culture is reduced, and viral material transferred to uninfected cells is less matured. At later time points, this defect is associated with a reduction in the productive infection of new target cells.
Conclusions: Our results highlight a previously unappreciated role of Nef during the viral replication cycle. Nef promotes HIV-1 Gag membrane localization and processing, and facilitates viral cell-to-cell transfer.
Figures




Similar articles
-
Human Polycomb group EED protein negatively affects HIV-1 assembly and release.Retrovirology. 2007 Jun 4;4:37. doi: 10.1186/1742-4690-4-37. Retrovirology. 2007. PMID: 17547741 Free PMC article.
-
Consequences of HLA-B*13-Associated Escape Mutations on HIV-1 Replication and Nef Function.J Virol. 2015 Nov;89(22):11557-71. doi: 10.1128/JVI.01955-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355081 Free PMC article.
-
Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly.J Virol. 2017 Feb 28;91(6):e02315-16. doi: 10.1128/JVI.02315-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053097 Free PMC article.
-
Rendezvous at Plasma Membrane: Cellular Lipids and tRNA Set up Sites of HIV-1 Particle Assembly and Incorporation of Host Transmembrane Proteins.Viruses. 2020 Jul 31;12(8):842. doi: 10.3390/v12080842. Viruses. 2020. PMID: 32752131 Free PMC article. Review.
-
Essential and supporting host cell factors for HIV-1 budding.Future Microbiol. 2011 Oct;6(10):1159-70. doi: 10.2217/fmb.11.100. Future Microbiol. 2011. PMID: 22004035 Review.
Cited by
-
HIV-1 cell-to-cell transmission and broadly neutralizing antibodies.Retrovirology. 2018 Jul 28;15(1):51. doi: 10.1186/s12977-018-0434-1. Retrovirology. 2018. PMID: 30055632 Free PMC article. Review.
-
HLA-DQB1*06 and breadth of Nef core region-specific T-cell response are associated with slow disease progression in antiretroviral therapy-naive Chinese HIV-1 subtype B patients.Hum Vaccin Immunother. 2017 Oct 3;13(10):2341-2347. doi: 10.1080/21645515.2017.1340138. Hum Vaccin Immunother. 2017. PMID: 28771107 Free PMC article.
-
HIV-1 sequences in the epidemic suggest an alternative pathway for the generation of the Long Terminal Repeats.Sci Rep. 2017 Oct 20;7(1):13715. doi: 10.1038/s41598-017-14135-z. Sci Rep. 2017. PMID: 29057964 Free PMC article.
-
Spotlight on HIV-1 Nef: SERINC3 and SERINC5 Identified as Restriction Factors Antagonized by the Pathogenesis Factor.Viruses. 2015 Dec 19;7(12):6730-8. doi: 10.3390/v7122970. Viruses. 2015. PMID: 26703715 Free PMC article.
-
Mechanisms for Cell-to-Cell Transmission of HIV-1.Front Immunol. 2018 Feb 19;9:260. doi: 10.3389/fimmu.2018.00260. eCollection 2018. Front Immunol. 2018. PMID: 29515578 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

