Phosphoinositides: tiny lipids with giant impact on cell regulation
- PMID: 23899561
- PMCID: PMC3962547
- DOI: 10.1152/physrev.00028.2012
Phosphoinositides: tiny lipids with giant impact on cell regulation
Abstract
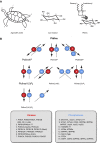
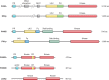
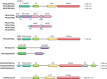
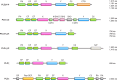
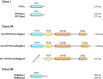
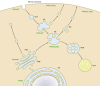
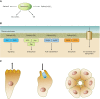
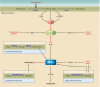
Phosphoinositides (PIs) make up only a small fraction of cellular phospholipids, yet they control almost all aspects of a cell's life and death. These lipids gained tremendous research interest as plasma membrane signaling molecules when discovered in the 1970s and 1980s. Research in the last 15 years has added a wide range of biological processes regulated by PIs, turning these lipids into one of the most universal signaling entities in eukaryotic cells. PIs control organelle biology by regulating vesicular trafficking, but they also modulate lipid distribution and metabolism via their close relationship with lipid transfer proteins. PIs regulate ion channels, pumps, and transporters and control both endocytic and exocytic processes. The nuclear phosphoinositides have grown from being an epiphenomenon to a research area of its own. As expected from such pleiotropic regulators, derangements of phosphoinositide metabolism are responsible for a number of human diseases ranging from rare genetic disorders to the most common ones such as cancer, obesity, and diabetes. Moreover, it is increasingly evident that a number of infectious agents hijack the PI regulatory systems of host cells for their intracellular movements, replication, and assembly. As a result, PI converting enzymes began to be noticed by pharmaceutical companies as potential therapeutic targets. This review is an attempt to give an overview of this enormous research field focusing on major developments in diverse areas of basic science linked to cellular physiology and disease.
Figures






References
-
- Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, Yuan JM, Zheng W, Dawsey SM, Dong LM, Lee MP, Ding T, Qiao YL, Gao YT, Koh WP, Xiang YB, Tang ZZ, Fan JH, Wang C, Wheeler W, Gail MH, Yeager M, Yuenger J, Hutchinson A, Jacobs KB, Giffen CA, Burdett L, Fraumeni JF, Jr, Tucker MA, Chow WH, Goldstein AM, Chanock SJ, Taylor PR. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet 42: 764–767, 2010 - PMC - PubMed
-
- Adamski FM, Timms KM, Shieh BH. A unique isoform of phospholipase Cbeta4 highly expressed in the cerebellum and eye. Biochim Biophys Acta 1444: 55–60, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

