Circadian rhythms, sleep deprivation, and human performance
- PMID: 23899598
- PMCID: PMC3963479
- DOI: 10.1016/B978-0-12-396971-2.00007-5
Circadian rhythms, sleep deprivation, and human performance
Abstract
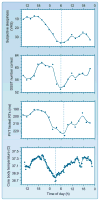
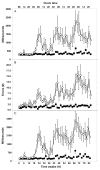
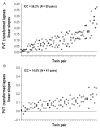
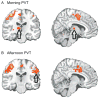
Much of the current science on, and mathematical modeling of, dynamic changes in human performance within and between days is dominated by the two-process model of sleep-wake regulation, which posits a neurobiological drive for sleep that varies homeostatically (increasing as a saturating exponential during wakefulness and decreasing in a like manner during sleep), and a circadian process that neurobiologically modulates both the homeostatic drive for sleep and waking alertness and performance. Endogenous circadian rhythms in neurobehavioral functions, including physiological alertness and cognitive performance, have been demonstrated using special laboratory protocols that reveal the interaction of the biological clock with the sleep homeostatic drive. Individual differences in circadian rhythms and genetic and other components underlying such differences also influence waking neurobehavioral functions. Both acute total sleep deprivation and chronic sleep restriction increase homeostatic sleep drive and degrade waking neurobehavioral functions as reflected in sleepiness, attention, cognitive speed, and memory. Recent evidence indicating a high degree of stability in neurobehavioral responses to sleep loss suggests that these trait-like individual differences are phenotypic and likely involve genetic components, including circadian genes. Recent experiments have revealed both sleep homeostatic and circadian effects on brain metabolism and neural activation. Investigation of the neural and genetic mechanisms underlying the dynamically complex interaction between sleep homeostasis and circadian systems is beginning. A key goal of this work is to identify biomarkers that accurately predict human performance in situations in which the circadian and sleep homeostatic systems are perturbed.
Keywords: Chronotype; Circadian rhythms; Genetics; Individual differences; Neuroimaging; Performance; Phenotype; Sleep deprivation; Sleep homeostasis; Two-process model.
© 2013, Elsevier Inc. All Rights Reserved.
Figures
References
-
- Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath. 2012;16:829–833. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

