Efficacy of exercise for menopausal symptoms: a randomized controlled trial
- PMID: 23899828
- PMCID: PMC3858421
- DOI: 10.1097/GME.0b013e31829e4089
Efficacy of exercise for menopausal symptoms: a randomized controlled trial
Abstract
Objective: This study aims to determine the efficacy of exercise training for alleviating vasomotor and other menopausal symptoms.
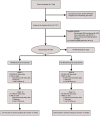
Methods: Late perimenopausal and postmenopausal sedentary women with frequent vasomotor symptoms (VMS) participated in a randomized controlled trial conducted in three sites: 106 women randomized to exercise and 142 women randomized to usual activity. The exercise intervention consisted of individual facility-based aerobic exercise training three times per week for 12 weeks. VMS frequency and bother were recorded on daily diaries at baseline and on weeks 6 and 12. Intent-to-treat analyses compared between-group differences in changes in VMS frequency and bother, sleep symptoms (Insomnia Severity Index and Pittsburgh Sleep Quality Index), and mood (Patient Health Questionnaire-8 and Generalized Anxiety Disorder-7 questionnaire).
Results: At the end of week 12, changes in VMS frequency in the exercise group (mean change, -2.4 VMS/d; 95% CI, -3.0 to -1.7) and VMS bother (mean change on a four-point scale, -0.5; 95% CI, -0.6 to -0.4) were not significantly different from those in the control group (-2.6 VMS/d; 95% CI, -3.2 to -2.0; P = 0.43; -0.5 points; 95% CI, -0.6 to -0.4; P = 0.75). The exercise group reported greater improvement in insomnia symptoms (P = 0.03), subjective sleep quality (P = 0.01), and depressive symptoms (P = 0.04), but differences were small and not statistically significant when P values were adjusted for multiple comparisons. Results were similar when considering treatment-adherent women only.
Conclusions: These findings provide strong evidence that 12 weeks of moderate-intensity aerobic exercise do not alleviate VMS but may result in small improvements in sleep quality, insomnia, and depression in midlife sedentary women.
Trial registration: ClinicalTrials.gov NCT01178892.
Figures
Comment in
-
Menopause strategies: Finding Lasting Answers for Symptoms and Health: eliminating hot flashes--still not a slam dunk!Menopause. 2014 Apr;21(4):321-2. doi: 10.1097/GME.0000000000000217. Menopause. 2014. PMID: 24552975 No abstract available.
-
Three alternative ways to treat VMS failed.Climacteric. 2014 Aug;17(4):512-3. Climacteric. 2014. PMID: 25147889 No abstract available.
-
To the Editor.Menopause. 2020 Mar;27(3):374. doi: 10.1097/GME.0000000000001483. Menopause. 2020. PMID: 32015262 No abstract available.
References
-
- Mayo Clinic Staff. Menopause. Available at http://www.mayoclinic.com/health/hot-fashes/DS01143/DSECTION=risk-factors.
-
- McAndrew LM, Napolitano MA, Albrecht A, Farrell NC, Marcus BH, Whiteley JA. When, why and for whom there is a relationship between physical activity and menopause symptoms. Maturitas. 2009 Oct 20;64(2):119–25. - PubMed
-
- Aiello EJ, Yasui Y, Tworoger SS, et al. Effect of a yearlong, moderate-intensity exercise intervention on the occurrence and severity of menopause symptoms in postmenopausal women. Menopause. 2004 Jul;11(4):382–8. - PubMed
-
- Wilbur J, Miller AM, McDevitt J, Wang E, Miller J. Menopausal status, moderate-intensity walking, and symptoms in midlife women. Res Theory Nurs Pract. 2005;19(2):163–80. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U01AG032659/AG/NIA NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 AG032682/AG/NIA NIH HHS/United States
- U01 AG032669/AG/NIA NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- U01 AG032659/AG/NIA NIH HHS/United States
- U01AG032699/AG/NIA NIH HHS/United States
- R01 AG048209/AG/NIA NIH HHS/United States
- U01AG032700/AG/NIA NIH HHS/United States
- U01 AG032699/AG/NIA NIH HHS/United States
- U01AG032669/AG/NIA NIH HHS/United States
- U01 AG032656/AG/NIA NIH HHS/United States
- U01AG032682/AG/NIA NIH HHS/United States
- U01 AG032700/AG/NIA NIH HHS/United States
- K01 HL108807/HL/NHLBI NIH HHS/United States