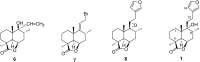
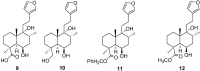
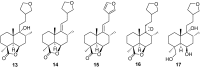
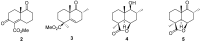Marrubiin
- PMID: 23899837
- PMCID: PMC6269822
- DOI: 10.3390/molecules18089049
Marrubiin
Abstract
The ethno-medicinal approach to drug discovery represents one of the most important sources of new and safe therapeutic agents to the challenges confronting modern medicine and daily life. Many of the traditionally important medicinal plants contain active molecules or ones that serve as precursors to biosynthesised secondary metabolites to which the biological activity could be attributed. Marrubiin is one such compound and is a potential valuable compound which exists in high concentrations in many traditionally important Lamiaceae species which have demonstrated excellent pharmacological properties with commendably high safety margins. Marrubiin's attributes include a low turnover, high stability and little catabolism, which are core characteristics required for therapeutic compounds and nutraceuticals of economic importance. In addition, marrubiin is considered a potential substrate for potent active compounds viz; marrubiinic acid, and marrubenol. The contribution of marrubiin to drug discovery thus needs to be put into prospective due to its ready availability, high potential applications and ease of modification. In this short review we highlight the most important chemical and pharmacological aspects reported on marrubiin since it was discovered.
Figures
References
-
- Abbondanza A.A., Breccia A. Crespi. Biosynthesis of labeled molecules: mechanism of biosynthetic reactions of precursors of furanicterpenes and phytosterol. Prepn. Bio-Med. Appl. Labeled Mol. Proc. Symp. Venice. 1964;67:95–101.
-
- Marrelli M., Conforti F., Rigano D., Formisano C., Bruno M., Senatore F., Menichini F. Cytotoxic properties of Marrubium globosum ssp. libanoticum and its bioactive components. Nat. Prod. Comm. 2013;8:567–569.
-
- Laonigro G., Lanzetta R., Parrilli M., Adinolfi M., Mangoni L. The configuration of the diterpene spiro ethers from Marrubium vulgare and from Leonotis leonurus. Gazz. Chim. Ital. 1979;109:145–150.
-
- Hussain J., Ullah R., Khan A., Mabood F., Shah M.R., Al-Harrasi A., Gilani A.H. Antispasmodic and Ca++ antagonist potential of marrubiin, a labdane type diterpene from Phlomis bracteosa. J. Pharmacy Res. 2011;4:178–180.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources