Gene therapy for cancer through adenovirus vector‑mediated expression of the Ad5 early region gene 1A based on loss of IGF2 imprinting
- PMID: 23900345
- PMCID: PMC3810216
- DOI: 10.3892/or.2013.2646
Gene therapy for cancer through adenovirus vector‑mediated expression of the Ad5 early region gene 1A based on loss of IGF2 imprinting
Abstract
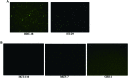
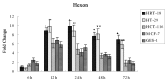
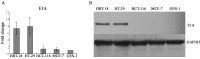
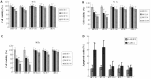
Loss of (genomic) imprinting (LOI) of the insulin-like growth factor 2 gene (IGF2) is a common epigenetic abnormality in many human cancers. IGF2 imprinting is regulated by differentially methylated domains (DMD) in the imprinting control region that is located between IGF2 and H19 on human chromosome 11. In the present study, combined expression of adenoviral vectors (Ad-EGFP and Ad-E1A) driven by H19 enhancer-DMD-H19 promoter complex was investigated and their effects on the tumor growth were assessed in vitro and in vivo. When infected with Ad-EGFP, the cancer cell lines with the LOI, such as HRT-18 and HT-29 cells, had the expression of the EGFP protein, whereas three cancer cell lines with the maintenance of imprinting (MOI) (HCT-116, MCF-7 and GES-1) had weak expression of EGFP. Furthermore, the expressed Ad-E1A significantly decreased cell viability and induced cell apoptosis only in HRT-18 and HT-29 cells in vitro, and effectively suppressed tumor development in HRT-18 and HT-29 xenograft in nude mice. It is concluded that this gene therapy vector is effective in the suppression of the growth of human colon cancer cells in vitro and in vivo, and that cancer gene therapy based on loss of IGF2 imprinting may prove to be a novel therapeutic option.
Figures



References
-
- Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–749. - PubMed
-
- Feinberg AP. Genomic imprinting and gene activation in cancer. Nat Genet. 1993;4:110–113. - PubMed
-
- Reik W, Constancia M, Dean W, Davies K, Bowden L, Murrell A, Feil R, Walter J, Kelsey G. Igf2 imprinting in development and disease. Int J Dev Biol. 2000;4:145–150. - PubMed
-
- Arney KL. H19 and Igf2-enhancing the confusion? Trends Genet. 2003;19:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

