Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1
- PMID: 23900607
- PMCID: PMC3771708
- DOI: 10.1167/iovs.13-11673
Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1
Abstract
Purpose: To study safety and efficacy of subretinal adeno-associated virus (AAV) vector AAV-Bbs1 injection for treatment of a mouse model of Bardet-Biedl syndrome type 1 (BBS1).
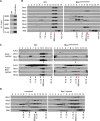
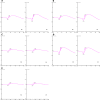
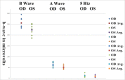
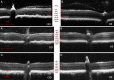
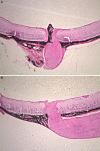
Methods: Constructs containing a wild-type (WT) Bbs1 gene with and without a FLAG tag in AAV2/5 vectors were generated. Viral genomes were delivered by subretinal injection to right eyes and sham injections to left eyes at postnatal day 30 (P30) to P60. Transgene expression and BBSome reconstitution were evaluated by immunohistochemistry and Western blotting following sucrose gradient ultracentrifugation. Retinal function was analyzed by electroretinogram (ERG) and structure by optical coherence tomography (OCT). Histology and immunohistochemistry were performed on selected eyes.
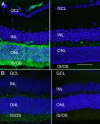
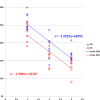
Results: Expression of FLAG-tagged Bbs1 was demonstrated in photoreceptor cells using antibody directed against the FLAG tag. Coinjection of AAV-GFP demonstrated transduction of 24% to 32% of the retina. Western blotting demonstrated BBS1 protein expression and reconstitution of the BBSome. ERG dark-adapted bright flash b-wave amplitudes were higher in AAV-Bbs1-injected eyes than in sham-injected fellow eyes in more than 50% of 19 animals. Anti-rhodopsin staining demonstrated improved localization of rhodopsin in AAV-Bbs1-treated eyes. WT retinas injected with AAV-Bbs1 with or without a FLAG tag showed outer retinal degeneration on ERG, OCT, and histology.
Conclusions: In a knock-in model of BBS1, subretinal delivery of AAV-Bbs1 rescues BBSome formation and rhodopsin localization, and shows a trend toward improved ERG. BBS is challenging to treat with gene therapy due to the stoichiometry of the BBSome protein complex and overexpression toxicity.
Keywords: AAV vector; Bardet Biedl syndrome; gene therapy; mouse model; retinal degeneration.
Figures







References
-
- Drack AV, Mullins RF, Seo S. RP syndromes: Bardet Biedl. In: Hartnett ME. Pediatric Retina. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
-
- Estrada-Cuzcano A, Roepman R, Cremers FP, den Hollander AI, Mans DA. Non-syndromic retinal ciliopathies: translating gene discovery into therapy. Hum Mol Genet. 2012; 21(R1): R111–R124 - PubMed
-
- Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007; 129: 1201–1213 - PubMed
-
- Coppieters F, Lefever S, Leroy BP, De Baere E. CEP290, a gene with many faces: mutation overview and presentation of CEP290base. Hum Mutat. 2010; 31: 1097–1108 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

