MK-2206 causes growth suppression and reduces neuroendocrine tumor marker production in medullary thyroid cancer through Akt inhibition
- PMID: 23900743
- PMCID: PMC3955957
- DOI: 10.1245/s10434-013-3168-2
MK-2206 causes growth suppression and reduces neuroendocrine tumor marker production in medullary thyroid cancer through Akt inhibition
Abstract
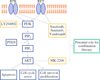
Background: Development of targeted therapies for medullary thyroid cancer (MTC) has focused on inhibition of the rearranged during transfection (RET) proto-oncogene. Akt has been demonstrated to be a downstream target of RET via the key mediator phosphoinositide-3-kinase. MK-2206 is an orally administered allosteric Akt inhibitor that has exhibited minimal toxicity in phase I trials. We explored the antitumor effects of this compound in MTC.
Methods: Human MTC-TT cells were treated with MK-2206 (0-20 μM) for 8 days. Assays for cell viability were performed at multiple time points with MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide). The mechanism of action, mechanism of growth inhibition, and production of neuroendocrine tumor markers were assessed with Western blot analysis.
Results: MK-2206 suppressed MTC cell proliferation in a dose-dependent manner (p ≤ 0.001). Levels of Akt phosphorylated at serine 473 declined with increasing doses of MK-2206, indicating successful Akt inhibition. The apoptotic proteins cleaved poly (ADP-ribose) polymerase and cleaved caspase-3 increased in a dose-dependent manner with MK-2206, while the apoptosis inhibitor survivin was markedly reduced. Importantly, the antitumor effects of MK-2206 were independent of RET inhibition, as the levels of RET protein were not blocked.
Conclusions: MK-2206 significantly suppresses MTC proliferation without RET inhibition. Given its high oral bioavailability and low toxicity profile, phase II studies with this drug alone or in combination with RET inhibitors are warranted.
Figures




References
-
- Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–547. - PubMed
-
- Pitt SC, Moley JF. Medullary, anaplastic, and metastatic cancers of the thyroid. Semin Oncol. 2010;37:567–579. - PubMed
-
- Chen H, Sippel RS, O’Dorisio MS, Vinik AI, Lloyd RV, Pacak K. North American Neuroendocrine Tumor Society. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39:775–783. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

