The biology of the germ line in echinoderms
- PMID: 23900765
- PMCID: PMC4102677
- DOI: 10.1002/mrd.22223
The biology of the germ line in echinoderms
Abstract
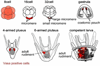
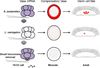
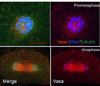
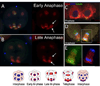
The formation of the germ line in an embryo marks a fresh round of reproductive potential. The developmental stage and location within the embryo where the primordial germ cells (PGCs) form, however, differs markedly among species. In many animals, the germ line is formed by an inherited mechanism, in which molecules made and selectively partitioned within the oocyte drive the early development of cells that acquire this material to a germ-line fate. In contrast, the germ line of other animals is fated by an inductive mechanism that involves signaling between cells that directs this specialized fate. In this review, we explore the mechanisms of germ-line determination in echinoderms, an early-branching sister group to the chordates. One member of the phylum, sea urchins, appears to use an inherited mechanism of germ-line formation, whereas their relatives, the sea stars, appear to use an inductive mechanism. We first integrate the experimental results currently available for germ-line determination in the sea urchin, for which considerable new information is available, and then broaden the investigation to the lesser-known mechanisms in sea stars and other echinoderms. Even with this limited insight, it appears that sea stars, and perhaps the majority of the echinoderm taxon, rely on inductive mechanisms for germ-line fate determination. This enables a strongly contrasted picture for germ-line determination in this phylum, but one for which transitions between different modes of germ-line determination might now be experimentally addressed.
© 2014 Wiley Periodicals, Inc.
Figures










References
-
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8:623–630. - PubMed
-
- Angerer LM, Angerer RC. Animal–vegetal axis patterning mechanisms in the early sea urchin embryo. Dev Biol. 2000;218:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

