A physiologically required G protein-coupled receptor (GPCR)-regulator of G protein signaling (RGS) interaction that compartmentalizes RGS activity
- PMID: 23900842
- PMCID: PMC3779728
- DOI: 10.1074/jbc.M113.497826
A physiologically required G protein-coupled receptor (GPCR)-regulator of G protein signaling (RGS) interaction that compartmentalizes RGS activity
Abstract
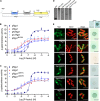
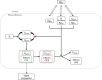
G protein-coupled receptors (GPCRs) can interact with regulator of G protein signaling (RGS) proteins. However, the effects of such interactions on signal transduction and their physiological relevance have been largely undetermined. Ligand-bound GPCRs initiate by promoting exchange of GDP for GTP on the Gα subunit of heterotrimeric G proteins. Signaling is terminated by hydrolysis of GTP to GDP through intrinsic GTPase activity of the Gα subunit, a reaction catalyzed by RGS proteins. Using yeast as a tool to study GPCR signaling in isolation, we define an interaction between the cognate GPCR (Mam2) and RGS (Rgs1), mapping the interaction domains. This reaction tethers Rgs1 at the plasma membrane and is essential for physiological signaling response. In vivo quantitative data inform the development of a kinetic model of the GTPase cycle, which extends previous attempts by including GPCR-RGS interactions. In vivo and in silico data confirm that GPCR-RGS interactions can impose an additional layer of regulation through mediating RGS subcellular localization to compartmentalize RGS activity within a cell, thus highlighting their importance as potential targets to modulate GPCR signaling pathways.
Keywords: Cell Compartmentation; Cell Signaling; G Protein-coupled Receptors (GPCR); GTPase; Kinetics; Mathematical Modeling; RGS Proteins; Signal Transduction.
Figures






References
-
- Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 - PubMed
-
- Sprang S. R. (1997) G proteins, effectors and GAPs. Structure and mechanism. Curr. Opin. Struct. Biol 7, 849–856 - PubMed
-
- Johnston C. A., Siderovski D. P. (2007) Receptor-mediated activation of heterotrimeric G-proteins. Current structural insights. Mol. Pharmacol. 72, 219–230 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

