Functional identification of the general acid and base in the dehydration step of indole-3-glycerol phosphate synthase catalysis
- PMID: 23900843
- PMCID: PMC3772182
- DOI: 10.1074/jbc.M113.487447
Functional identification of the general acid and base in the dehydration step of indole-3-glycerol phosphate synthase catalysis
Abstract
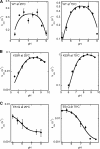
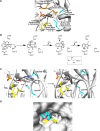
The tryptophan biosynthetic enzyme indole-3-glycerol phosphate synthase is a proposed target for new antimicrobials and is a favored starting framework in enzyme engineering studies. Forty years ago, Parry proposed that the enzyme mechanism proceeds through two intermediates in a series of condensation, decarboxylation, and dehydration steps. X-ray crystal structures have suggested that Lys-110 (numbering according to the Sulfolobus solfataricus enzyme) behaves as a general acid both in the condensation and dehydration steps, but did not reveal an efficient pathway for the reprotonation of this critical residue. Our mutagenesis and kinetic experiments suggest an alternative mechanism whereby Lys-110 acts as a general acid in the condensation step, but another invariant residue, Lys-53, acts as the general acid in the dehydration step. These studies also indicate that the conserved residue Glu-51 acts as the general base in the dehydration step. The revised mechanism effectively divides the active site into discrete regions where the catalytic surfaces containing Lys-110 and Lys-53/Glu-51 catalyze the ring closure (i.e. condensation and decarboxylation) and dehydration steps, respectively. These results can be leveraged toward the development of novel inhibitors against this validated antimicrobial target and toward the rational engineering of the enzyme to produce indole derivatives that are highly prized by the pharmaceutical and agricultural industries.
Keywords: Bacterial Metabolism; Drug Development; Enzyme Kinetics; Enzyme Mechanisms; Protein Engineering; Tryptophan.
Figures
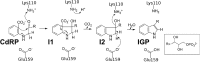

References
-
- Barden T. C. (2010) Indoles: industrial, agricultural and over-the-counter uses. Top. Hetercycl. Chem. 26, 31–46
-
- Humphrey G. R., Kuethe J. T. (2006) Practical methodologies for the synthesis of indoles. Chem. Rev. 106, 2875–2911 - PubMed
-
- Shen H., Wang F., Zhang Y., Huang Q., Xu S., Hu H., Yue J., Wang H. (2009) A novel inhibitor of indole-3-glycerol phosphate synthase with activity against multidrug-resistant Mycobacterium tuberculosis. FEBS J. 276, 144–154 - PubMed
-
- Shen H., Yang E., Wang F., Jin R., Xu S., Huang Q., Wang H. (2010) Altered protein expression patterns of Mycobacterium tuberculosis induced by ATB107. J. Microbiol. 48, 337–346 - PubMed
-
- Czekster C. M., Neto B. A. D., Lapis A. A. M., Dupont J., Santos D. S., Basso L. A. (2009) Steady-state kinetics of indole-3-glycerol phosphate synthase from Mycobacterium tuberculosis. Arch. Biochem. Biophys. 486, 19–26 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

