Toward real-time temperature monitoring in fat and aqueous tissue during magnetic resonance-guided high-intensity focused ultrasound using a three-dimensional proton resonance frequency T1 method
- PMID: 23901014
- PMCID: PMC5432131
- DOI: 10.1002/mrm.24900
Toward real-time temperature monitoring in fat and aqueous tissue during magnetic resonance-guided high-intensity focused ultrasound using a three-dimensional proton resonance frequency T1 method
Abstract
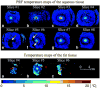
Purpose: To present a three-dimensional (3D) segmented echoplanar imaging (EPI) pulse sequence implementation that provides simultaneously the proton resonance frequency shift temperature of aqueous tissue and the longitudinal relaxation time (T1 ) of fat during thermal ablation.
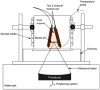
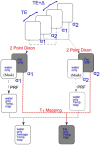
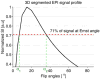
Methods: The hybrid sequence was implemented by combining a 3D segmented flyback EPI sequence, the extended two-point Dixon fat and water separation, and the double flip angle T1 mapping techniques. High-intensity focused ultrasound (HIFU) heating experiments were performed at three different acoustic powers on excised human breast fat embedded in ex vivo porcine muscle. Furthermore, T1 calibrations with temperature in four different excised breast fat samples were performed, yielding an estimate of the average and variation of dT1 /dT across subjects.
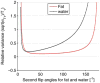
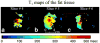
Results: The water only images were used to mask the complex original data before computing the proton resonance frequency shift. T1 values were calculated from the fat-only images. The relative temperature coefficients were found in five fat tissue samples from different patients and ranged from 1.2% to 2.6%/°C.
Conclusion: The results demonstrate the capability of real-time simultaneous temperature mapping in aqueous tissue and T1 mapping in fat during HIFU ablation, providing a potential tool for treatment monitoring in organs with large fat content, such as the breast.
Keywords: aqueous tissue; fat; temperature.
Copyright © 2013 Wiley Periodicals, Inc.
Figures



References
-
- Hynynen K, McDannold N, Mulkern RV, Jolesz FA. Temperature monitoring in fat with MRI. Magn Reson Med. 2000;43:901–904. - PubMed
-
- Graham SJ, Bronskill MJ, Henkelman RM. Time and temperature dependence of MR parameters during thermal coagulation of ex vivo rabbit muscle. Magn Reson Med. 1998;39:198–203. - PubMed
-
- Kuroda KOK, Mulkern RV, Jolesz FA. Optimization of chemical shift selective suppression of fat. Magn Reson Med. 1996;40:505–510. - PubMed
-
- Vandeweyer E, Hertens D. Quantification of glands and fat in breast tissue: an experimental determination. Ann Anat. 2002;184:181–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

