Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults
- PMID: 23901074
- PMCID: PMC4224242
- DOI: 10.1093/cercor/bht188
Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults
Abstract
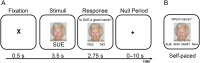
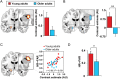
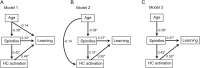
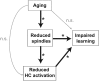
A hallmark feature of cognitive aging is a decline in the ability to form new memories. Parallel to these cognitive impairments are marked disruptions in sleep physiology. Despite recent evidence in young adults establishing a role for sleep spindles in restoring hippocampal-dependent memory formation, the possibility that disrupted sleep physiology contributes to age-related decline in hippocampal-dependent learning remains unknown. Here, we demonstrate that reduced prefrontal sleep spindles by over 40% in older adults statistically mediates the effects of old age on next day episodic learning, such that the degree of impaired episodic learning is explained by the extent of impoverished prefrontal sleep spindles. In addition, prefrontal spindles significantly predicted the magnitude of impaired next day hippocampal activation, thereby determining the influence of spindles on post-sleep learning capacity. These data support the hypothesis that disrupted sleep physiology contributes to age-related cognitive decline in later life, the consequence of which has significant treatment intervention potential.
Keywords: aging; fMRI; hippocampus; learning; sleep.
© The Author 2013. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. - PubMed
-
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B Method. 1995;57:289–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

