Serum drug concentrations predictive of pulmonary tuberculosis outcomes
- PMID: 23901086
- PMCID: PMC3789573
- DOI: 10.1093/infdis/jit352
Serum drug concentrations predictive of pulmonary tuberculosis outcomes
Abstract
Background: Based on a hollow-fiber system model of tuberculosis, we hypothesize that microbiologic failure and acquired drug resistance are primarily driven by low drug concentrations that result from pharmacokinetic variability.
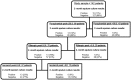
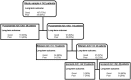
Methods: Clinical and pharmacokinetic data were prospectively collected from 142 tuberculosis patients in Western Cape, South Africa. Compartmental pharmacokinetic parameters of isoniazid, rifampin, and pyrazinamide were identified for each patient. Patients were then followed for up to 2 years. Classification and regression tree analysis was used to identify and rank clinical predictors of poor long-term outcome such as microbiologic failure or death, or relapse.
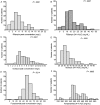
Results: Drug concentrations and pharmacokinetics varied widely between patients. Poor long-term outcomes were encountered in 35 (25%) patients. The 3 top predictors of poor long-term outcome, by rank of importance, were a pyrazinamide 24-hour area under the concentration-time curve (AUC) ≤ 363 mg·h/L, rifampin AUC ≤ 13 mg·h/L, and isoniazid AUC ≤ 52 mg·h/L. Poor outcomes were encountered in 32/78 patients with the AUC of at least 1 drug below the identified threshold vs 3/64 without (odds ratio = 14.14; 95% confidence interval, 4.08-49.08). Low rifampin and isoniazid peak and AUC concentrations preceded all cases of acquired drug resistance.
Conclusions: Low drug AUCs are predictive of clinical outcomes in tuberculosis patients.
Keywords: classification and regression tree analysis; drug concentrations; hollow-fiber system; nonlinear systems; outcomes; pharmacokinetic variability; tuberculosis.
Figures
References
-
- Visser ME, Grewal HM, Swart EC, et al. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. Am J Clin Nutr. 2011;93:93–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

