Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues
- PMID: 23901099
- PMCID: PMC3746876
- DOI: 10.1073/pnas.1304749110
Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues
Abstract
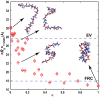
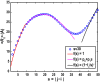
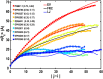
The functions of intrinsically disordered proteins (IDPs) are governed by relationships between information encoded in their amino acid sequences and the ensembles of conformations that they sample as autonomous units. Most IDPs are polyampholytes, with sequences that include both positively and negatively charged residues. Accordingly, we focus here on the sequence-ensemble relationships of polyampholytic IDPs. The fraction of charged residues discriminates between weak and strong polyampholytes. Using atomistic simulations, we show that weak polyampholytes form globules, whereas the conformational preferences of strong polyampholytes are determined by a combination of fraction of charged residues values and the linear sequence distributions of oppositely charged residues. We quantify the latter using a patterning parameter κ that lies between zero and one. The value of κ is low for well-mixed sequences, and in these sequences, intrachain electrostatic repulsions and attractions are counterbalanced, leading to the unmasking of preferences for conformations that resemble either self-avoiding random walks or generic Flory random coils. Segregation of oppositely charged residues within linear sequences leads to high κ-values and preferences for hairpin-like conformations caused by long-range electrostatic attractions induced by conformational fluctuations. We propose a scaling theory to explain the sequence-encoded conformational properties of strong polyampholytes. We show that naturally occurring strong polyampholytes have low κ-values, and this feature implies a selection for random coil ensembles. The design of sequences with different κ-values demonstrably alters the conformational preferences of polyampholytic IDPs, and this ability could become a useful tool for enabling direct inquiries into connections between sequence-ensemble relationships and functions of IDPs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

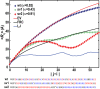
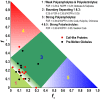
References
-
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6(3):197–208. - PubMed
-
- Tantos A, Han KH, Tompa P. Intrinsic disorder in cell signaling and gene transcription. Mol Cell Endocrinol. 2012;348(2):457–465. - PubMed
-
- Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269(1):2–12. - PubMed
-
- Bright JN, Woolf TB, Hoh JH. Predicting properties of intrinsically unstructured proteins. Prog Biophys Mol Biol. 2001;76(3):131–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

