Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain
- PMID: 23901103
- PMCID: PMC3746859
- DOI: 10.1073/pnas.1309275110
Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain
Abstract
P-glycoprotein (P-gp) is one of the best-known mediators of drug efflux-based multidrug resistance in many cancers. This validated therapeutic target is a prototypic, plasma membrane resident ATP-Binding Cassette transporter that pumps xenobiotic compounds out of cells. The large, polyspecific drug-binding pocket of P-gp recognizes a variety of structurally unrelated compounds. The transport of these drugs across the membrane is coincident with changes in the size and shape of this pocket during the course of the transport cycle. Here, we present the crystal structures of three inward-facing conformations of mouse P-gp derived from two different crystal forms. One structure has a nanobody bound to the C-terminal side of the first nucleotide-binding domain. This nanobody strongly inhibits the ATP hydrolysis activity of mouse P-gp by hindering the formation of a dimeric complex between the ATP-binding domains, which is essential for nucleotide hydrolysis. Together, these inward-facing conformational snapshots of P-gp demonstrate a range of flexibility exhibited by this transporter, which is likely an essential feature for the binding and transport of large, diverse substrates. The nanobody-bound structure also reveals a unique epitope on P-gp.
Keywords: ABC transporter; membrane protein structure; nanobody-transporter complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
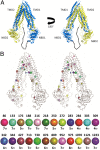

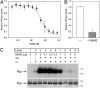
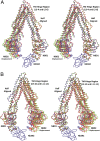
References
-
- Cascorbi I (2011) P-glycoprotein: Tissue distribution, substrates, and functional consequences of genetic variations. Handb Exp Pharmacol (201):261–283. - PubMed
-
- Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev. 2009;109(7):2989–3011. - PubMed
-
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv Drug Deliv Rev. 2003;55(1):3–29. - PubMed
-
- Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006;580(4):998–1009. - PubMed
-
- Marquez B, Van Bambeke F. ABC multidrug transporters: Target for modulation of drug pharmacokinetics and drug-drug interactions. Curr Drug Targets. 2011;12(5):600–620. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

