Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells
- PMID: 23901105
- PMCID: PMC3746888
- DOI: 10.1073/pnas.1300963110
Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells
Abstract
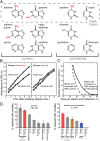
Primordial cells presumably combined RNAs, which functioned as catalysts and carriers of genetic information, with an encapsulating membrane of aggregated amphiphilic molecules. Major questions regarding this hypothesis include how the four bases and the sugar in RNA were selected from a mixture of prebiotic compounds and colocalized with such membranes, and how the membranes were stabilized against flocculation in salt water. To address these questions, we explored the possibility that aggregates of decanoic acid, a prebiotic amphiphile, interact with the bases and sugar found in RNA. We found that these bases, as well as some but not all related bases, bind to decanoic acid aggregates. Moreover, both the bases and ribose inhibit flocculation of decanoic acid by salt. The extent of inhibition by the bases correlates with the extent of their binding, and ribose inhibits to a greater extent than three similar sugars. Finally, the stabilizing effects of a base and ribose are additive. Thus, aggregates of a prebiotic amphiphile bind certain heterocyclic bases and sugars, including those found in RNA, and this binding stabilizes the aggregates against salt. These mutually reinforcing mechanisms might have driven the emergence of protocells.
Keywords: fatty acids; micelles; nucleosides; origin of life; vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Step toward Molecular Evolution of RNA: Ribose Binds to Prebiotic Fatty Acid Membranes, and Nucleosides Bind Better than Individual Bases Do.Chembiochem. 2020 Oct 1;21(19):2764-2767. doi: 10.1002/cbic.202000260. Epub 2020 Jun 5. Chembiochem. 2020. PMID: 32358921 Free PMC article.
-
Prebiotic Vesicle Formation and the Necessity of Salts.Orig Life Evol Biosph. 2016 Jun;46(2-3):215-22. doi: 10.1007/s11084-015-9476-8. Epub 2015 Nov 21. Orig Life Evol Biosph. 2016. PMID: 26590931
-
Prebiotic Membranes and Micelles Do Not Inhibit Peptide Formation During Dehydration.Chembiochem. 2022 Feb 4;23(3):e202100614. doi: 10.1002/cbic.202100614. Epub 2021 Dec 20. Chembiochem. 2022. PMID: 34881485 Free PMC article.
-
A Self-Assembled Aggregate Composed of a Fatty Acid Membrane and the Building Blocks of Biological Polymers Provides a First Step in the Emergence of Protocells.Life (Basel). 2016 Aug 11;6(3):33. doi: 10.3390/life6030033. Life (Basel). 2016. PMID: 27529283 Free PMC article. Review.
-
Prebiotic RNA self-assembling and the origin of life: Mechanistic and molecular modeling rationale for explaining the prebiotic origin and replication of RNA.Acta Histochem. 2025 Mar;127(1):152226. doi: 10.1016/j.acthis.2024.152226. Epub 2025 Jan 8. Acta Histochem. 2025. PMID: 39788859 Review.
Cited by
-
Synthetic biology outside the cell: linking computational tools to cell-free systems.Front Bioeng Biotechnol. 2014 Dec 9;2:66. doi: 10.3389/fbioe.2014.00066. eCollection 2014. Front Bioeng Biotechnol. 2014. PMID: 25538941 Free PMC article. Review.
-
Prebiotic amino acids bind to and stabilize prebiotic fatty acid membranes.Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17239-17244. doi: 10.1073/pnas.1900275116. Epub 2019 Aug 12. Proc Natl Acad Sci U S A. 2019. PMID: 31405964 Free PMC article.
-
Plausible Sources of Membrane-Forming Fatty Acids on the Early Earth: A Review of the Literature and an Estimation of Amounts.ACS Earth Space Chem. 2022 Dec 22;7(1):11-27. doi: 10.1021/acsearthspacechem.2c00168. eCollection 2023 Jan 19. ACS Earth Space Chem. 2022. PMID: 36704178 Free PMC article. Review.
-
Synergism and mutualism in non-enzymatic RNA polymerization.Life (Basel). 2014 Nov 3;4(4):598-620. doi: 10.3390/life4040598. Life (Basel). 2014. PMID: 25370531 Free PMC article. Review.
-
Selection of Prebiotic Molecules in Amphiphilic Environments.Life (Basel). 2017 Jan 7;7(1):3. doi: 10.3390/life7010003. Life (Basel). 2017. PMID: 28067845 Free PMC article.
References
-
- Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459(7244):239–242. - PubMed
-
- Deamer D, Dworkin JP, Sandford SA, Bernstein MP, Allamandola LJ. The first cell membranes. Astrobiology. 2002;2(4):371–381. - PubMed
-
- Apel CL, Deamer DW, Mautner MN. Self-assembled vesicles of monocarboxylic acids and alcohols: Conditions for stability and for the encapsulation of biopolymers. Biochim Biophys Acta. 2002;1559(1):1–9. - PubMed

