miR-29 acts as a decoy in sarcomas to protect the tumor suppressor A20 mRNA from degradation by HuR
- PMID: 23901138
- PMCID: PMC3885907
- DOI: 10.1126/scisignal.2004177
miR-29 acts as a decoy in sarcomas to protect the tumor suppressor A20 mRNA from degradation by HuR
Erratum in
- Sci Signal. 2013 Sep 10;6(292):er6. Balkhi, Mumtaz Y [corrected to Balkhi, M Y]
Abstract
In sarcoma, the activity of NF-κB (nuclear factor κB) reduces the abundance of the microRNA (miRNA) miR-29. The tumor suppressor A20 [also known as TNFAIP3 (tumor necrosis factor-α-induced protein 3)] inhibits an upstream activator of NF-κB and is often mutated in lymphomas. In a panel of human sarcoma cell lines, we found that the activation of NF-κB was increased and, although the abundance of A20 protein and mRNA was decreased, the gene encoding A20 was rarely mutated. The 3' untranslated region (UTR) of A20 mRNA has conserved binding sites for both of the miRNAs miR-29 and miR-125. Whereas the expression of miR-125 was increased in human sarcoma tissue, that of miR-29 was decreased in most samples. Overexpression of miR-125 decreased the abundance of A20 mRNA, whereas reconstituting miR-29 in sarcoma cell lines increased the abundance of A20 mRNA and protein. By interacting directly with the RNA binding protein HuR (human antigen R; also known as ELAVL1), miR-29 prevented HuR from binding to the A20 3'UTR and recruiting the RNA degradation complex RISC (RNA-induced silencing complex), suggesting that miR-29 can act as a decoy for HuR, thus protecting A20 transcripts. Decreased miR-29 and A20 abundance in sarcomas correlated with increased activity of NF-κB and decreased expression of genes associated with differentiation. Together, the findings reveal a unique role of miR-29 and suggest that its absence may contribute to sarcoma tumorigenesis.
Conflict of interest statement
Figures
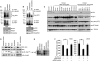


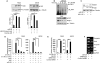


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

