Wnt and the cancer niche: paracrine interactions with gastrointestinal cancer cells undergoing asymmetric cell division
- PMID: 23901343
- PMCID: PMC3726705
- DOI: 10.7150/jca.6896
Wnt and the cancer niche: paracrine interactions with gastrointestinal cancer cells undergoing asymmetric cell division
Abstract
Objective: Stem-like cancer cells contribute to cancer initiation and maintenance. Stem cells can self-renew by asymmetric cell division (ACD). ACD with non-random chromosomal cosegregation (ACD-NRCC) is one possible self-renewal mechanism. There is a paucity of evidence supporting ACD-NRCC in human cancer. Our aim was to investigate ACD-NRCC and its potential interactions with the cancer niche (microenvironment) in gastrointestinal cancers.
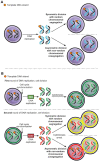
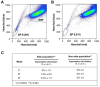
Design: We used DNA double and single labeling approaches with FACS to isolate live cells undergoing ACD-NRCC.
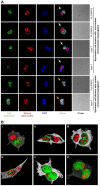
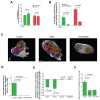
Results: Gastrointestinal cancers contain rare subpopulations of cells capable of ACD-NRCC. ACD-NRCC was detected preferentially in subpopulations of cells previously suggested to be stem-like/tumor-initiating cancer cells. ACD-NRCC was independent of cell-to-cell contact, and was regulated by the cancer niche in a heat-sensitive paracrine fashion. Wnt pathway genes and proteins are differentially expressed in cells undergoing ACD-NRCC vs. symmetric cell division. Blocking the Wnt pathway with IWP2 (WNT antagonist) or siRNA-TCF4 resulted in suppression of ACD-NRCC. However, using a Wnt-agonist did not increase the relative proportion of cells undergoing ACD-NRCC.
Conclusion: Gastrointestinal cancers contain subpopulations of cells capable of ACD-NRCC. Here we show for the first time that ACD-NRCC can be regulated by the Wnt pathway, and by the cancer niche in a paracrine fashion. However, whether ACD-NRCC is exclusively associated with stem-like cancer cells remains to be determined. Further study of these findings might generate novel insights into stem cell and cancer biology. Targeting the mechanism of ACD-NRCC might engender novel approaches for cancer therapy.
Keywords: Asymmetric Cell Division; Cancer Stem Cells; Microenvironment.; Non-Random Chromosomal Cosegregation.
Conflict of interest statement
Competing interest: The authors indicate no potential conflicts of interest.
Figures


References
-
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. - PubMed
-
- Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–43. - PubMed
-
- Fei JF, Huttner WB. Nonselective sister chromatid segregation in mouse embryonic neocortical precursor cells. Cereb Cortex. 2009;19(Suppl 1):i49–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

