Inhibition of sodium-linked glucose reabsorption normalizes diabetes-induced glomerular hyperfiltration in conscious adenosine A₁-receptor deficient mice
- PMID: 23901799
- PMCID: PMC3909021
- DOI: 10.1111/apha.12152
Inhibition of sodium-linked glucose reabsorption normalizes diabetes-induced glomerular hyperfiltration in conscious adenosine A₁-receptor deficient mice
Abstract
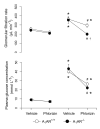
Aim: Glomerular hyperfiltration is commonly observed in diabetics early after the onset of the disease and predicts the progression of nephropathy. Sustained hyperglycaemia is also closely associated with kidney hypertrophy and increased electrolyte and glucose reabsorption in the proximal tubule. In this study, we investigated the role of the increased tubular sodium/glucose cotransport for diabetes-induced glomerular hyperfiltration. To eliminate any potential confounding effect of the tubuloglomerular feedback (TGF) mechanism, we used adenosine A₁-receptor deficient (A1AR(-/-)) mice known to lack a functional TGF mechanism and compared the results to corresponding wild-type animals (A1AR(+/+)).
Methods: Diabetes was induced by an intravenous bolus injection of alloxan. Glomerular filtration rate (GFR) was determined in conscious mice by a single bolus injection of inulin. The sodium/glucose cotransporters were inhibited by phlorizin 30 min prior to GFR measurements.
Results: Normoglycaemic animals had a similar GFR independent of genotype (A₁AR(+/+) 233 ± 11 vs. A₁AR(-/-) 241 ± 25 μL min(-1)), and induction of diabetes resulted in glomerular hyperfiltration in both groups (A₁AR(+/+) 380 ± 25 vs. A₁AR(-/-) 336 ± 35 μL min(-1); both P < 0.05). Phlorizin had no effect on GFR in normoglycaemic mice, whereas it reduced GFR in both genotypes during diabetes (A₁AR(+/+) 365 ± 18 to 295 ± 19, A₁AR(-/-) 354 ± 38 to 199 ± 15 μL min(-1); both P < 0.05). Notably, the reduction was more pronounced in the A₁AR(-/-) (P < 0.05).
Conclusion: This study demonstrates that increased tubular sodium/glucose reabsorption is important for diabetes-induced hyperfiltration, and that the TGF mechanism is not involved in these alterations, but rather functions to reduce any deviations from a new set-point.
Keywords: adenosine; diabetes mellitus; glomerular hyperfiltration; tubuloglomerular feedback.
© 2013 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd.
Figures

Comment in
-
The maturing relationship between tubules and glomeruli in diabetes mellitus.Acta Physiol (Oxf). 2014 Feb;210(2):233-5. doi: 10.1111/apha.12161. Epub 2013 Oct 7. Acta Physiol (Oxf). 2014. PMID: 23998920 No abstract available.
References
-
- Bank N. Mechanisms of diabetic hyperfiltration. Kidney Int. 1991;40:792–807. - PubMed
-
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–7. - PubMed
-
- Ditzel J, Schwartz M. Abnormally increased glomerular filtration rate in short-term insulin-treated diabetic subjects. Diabetes. 1967;16:264–7. - PubMed
-
- Freitas HS, Anhe GF, Melo KF, Okamoto MM, Oliveira-Souza M, Bordin S, Machado UF. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology. 2008;149:717–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

