Hemojuvelin modulates iron stress during acute kidney injury: improved by furin inhibitor
- PMID: 23901875
- PMCID: PMC3934545
- DOI: 10.1089/ars.2013.5366
Hemojuvelin modulates iron stress during acute kidney injury: improved by furin inhibitor
Abstract
Aims: Free iron plays an important role in the pathogenesis of acute kidney injury (AKI) via the formation of hydroxyl radicals. Systemic iron homeostasis is controlled by the hemojuvelin-hepcidin-ferroportin axis in the liver, but less is known about this role in AKI.
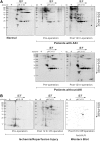
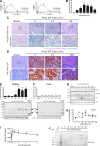
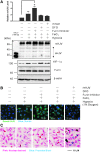
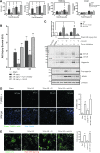
Results: By proteomics, we identified a 42 kDa soluble hemojuvelin (sHJV), processed by furin protease from membrane-bound hemojuvelin (mHJV), in the urine during AKI after cardiac surgery. Biopsies from human and mouse specimens with AKI confirm that HJV is extensively increased in renal tubules. Iron overload enhanced the expression of hemojuvelin-hepcidin signaling pathway. The furin inhibitor (FI) decreases furin-mediated proteolytic cleavage of mHJV into sHJV and augments the mHJV/sHJV ratio after iron overload with hypoxia condition. The FI could reduce renal tubule apoptosis, stabilize hypoxic induced factor-1, prevent the accumulation of iron in the kidney, and further ameliorate ischemic-reperfusion injury. mHJV is associated with decreasing total kidney iron, secreting hepcidin, and promoting the degradation of ferroportin at AKI, whereas sHJV does the opposite.
Innovation: This study suggests the ratio of mHJV/sHJV affects the iron deposition during acute kidney injury and sHJV could be an early biomarker of AKI.
Conclusion: Our findings link endogenous HJV inextricably with renal iron homeostasis for the first time, add new significance to early predict AKI, and identify novel therapeutic targets to reduce the severity of AKI using the FI.
Figures



References
-
- Baliga R, Zhang ZW, Baliga M, and Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 49: 362–369, 1996 - PubMed
-
- This reference has been deleted.
-
- Baliga R, Zhang ZW, and Shah SV. Role of cytochrome P-450 in hydrogen peroxide-induced cytotoxicity to LLC-PK1 cells. Kidney Int 50: 1118–1124, 1996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

