Solution structure and dynamics of human hemoglobin in the carbonmonoxy form
- PMID: 23901897
- PMCID: PMC4013309
- DOI: 10.1021/bi4005683
Solution structure and dynamics of human hemoglobin in the carbonmonoxy form
Abstract
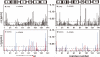
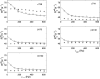
The solution structure of human adult carbonmonoxy hemoglobin (HbCO A) was refined using stereospecifically assigned methyl groups and residual dipolar couplings based on our previous nuclear magnetic resonance structure. The tertiary structures of individual chains were found to be very similar to the X-ray structures, while the quaternary structures in solution at low salt concentrations resembled the X-ray R structure more than the R2 structure. On the basis of chemical shift perturbation by inositol hexaphosphate (IHP) titration and docking, we identified five possible IHP binding sites in HbCO A. Amide-water proton exchange experiments demonstrated that αThr38 located in the α1β2 interface and several loop regions in both α- and β-chains were dynamic on the subsecond time scale. Side chain methyl dynamics revealed that methyl groups in the α1β2 interface were dynamic, but those in the α1β1 interface were quite rigid on the nanosecond to picosecond and millisecond to microsecond time scales. All the data strongly suggest a dynamic α1β2 interface that allows conformational changes among different forms (like T, R, and R2) easily in solution. Binding of IHP to HbCO A induced small structural and dynamic changes in the α1β2 interface and the regions around the hemes but did not increase the conformational entropy of HbCO A. The binding also caused conformational changes on the millisecond time scale, very likely arising from the relative motion of the α1β1 dimer with respect to the α2β2 dimer. Heterotropic effectors like IHP may change the oxygen affinity of Hb through modulating the relative motion of the two dimers and then further altering the structure of heme binding regions.
Figures
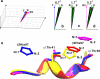



References
-
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965;12:88–118. - PubMed
-
- Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. - PubMed
-
- Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J. Biol. Chem. 1992;267:17248–17256. - PubMed
-
- Safo MK, Abraham DJ. The enigma of the liganded hemoglobin end state: a novel quaternary structure of human carbonmonoxy hemoglobin. Biochemistry. 2005;44:8347–8359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

