Dual delivery of rhPDGF-BB and bone marrow mesenchymal stromal cells expressing the BMP2 gene enhance bone formation in a critical-sized defect model
- PMID: 23901900
- PMCID: PMC3807533
- DOI: 10.1089/ten.tea.2012.0648
Dual delivery of rhPDGF-BB and bone marrow mesenchymal stromal cells expressing the BMP2 gene enhance bone formation in a critical-sized defect model
Abstract
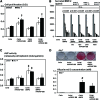
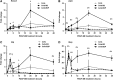
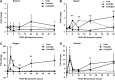
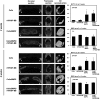
Bone tissue healing is a dynamic, orchestrated process that relies on multiple growth factors and cell types. Platelet-derived growth factor-BB (PDGF-BB) is released from platelets at wound sites and induces cellular migration and proliferation necessary for bone regeneration in the early healing process. Bone morphogenetic protein-2 (BMP-2), the most potent osteogenic differentiation inducer, directs new bone formation at the sites of bone defects. This study evaluated a combinatorial treatment protocol of PDGF-BB and BMP-2 on bone healing in a critical-sized defect model. To mimic the bone tissue healing process, a dual delivery approach was designed to deliver the rhPDGF-BB protein transiently during the early healing phase, whereas BMP-2 was supplied by rat bone marrow stromal cells (BMSCs) transfected with an adenoviral vector containing the BMP2 gene (AdBMP2) for prolonged release throughout the healing process. In in vitro experiments, the dual delivery of rhPDGF-BB and BMP2 significantly enhanced cell proliferation. However, the osteogenic differentiation of BMSCs was significantly suppressed even though the amount of BMP-2 secreted by the AdBMP2-transfected BMSCs was not significantly affected by the rhPDGF-BB treatment. In addition, dual delivery inhibited the mRNA expression of BMP receptor type II and Noggin in BMSCs. In in vivo experiments, critical-sized calvarial defects in rats showed enhanced bone regeneration by dual delivery of autologous AdBMP2-transfected BMSCs and rhPDGF-BB in both the amount of new bone formed and the bone mineral density. These enhancements in bone regeneration were greater than those observed in the group treated with AdBMP2-transfected BMSCs alone. In conclusion, the dual delivery of rhPDGF-BB and AdBMP2-transfected BMSCs improved the quality of the regenerated bone, possibly due to the modulation of PDGF-BB on BMP-2-induced osteogenesis.
Figures

Similar articles
-
The healing of critical-size calvarial bone defects in rat with rhPDGF-BB, BMSCs, and β-TCP scaffolds.J Mater Sci Mater Med. 2012 Apr;23(4):1073-84. doi: 10.1007/s10856-012-4558-x. Epub 2012 Feb 7. J Mater Sci Mater Med. 2012. PMID: 22311076
-
rhPDGF-BB promotes proliferation and osteogenic differentiation of bone marrow stromal cells from streptozotocin-induced diabetic rats through ERK pathway.Biomed Res Int. 2014;2014:637415. doi: 10.1155/2014/637415. Epub 2014 Jan 29. Biomed Res Int. 2014. PMID: 24605332 Free PMC article.
-
rhPDGF-BB via ERK pathway osteogenesis and adipogenesis balancing in ADSCs for critical-sized calvarial defect repair.Tissue Eng Part A. 2014 Dec;20(23-24):3303-13. doi: 10.1089/ten.TEA.2013.0556. Tissue Eng Part A. 2014. PMID: 24568547
-
Twenty-five years of recombinant human growth factors rhPDGF-BB and rhBMP-2 in oral hard and soft tissue regeneration.Periodontol 2000. 2024 Feb;94(1):483-509. doi: 10.1111/prd.12522. Epub 2023 Sep 8. Periodontol 2000. 2024. PMID: 37681552 Review.
-
The role of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) in orthopaedic bone repair and regeneration.Curr Pharm Des. 2013;19(19):3384-90. doi: 10.2174/1381612811319190005. Curr Pharm Des. 2013. PMID: 23432673 Review.
Cited by
-
Platelet-Derived Growth Factor BB Enhances Osteogenesis of Adipose-Derived But Not Bone Marrow-Derived Mesenchymal Stromal/Stem Cells.Stem Cells. 2015 Sep;33(9):2773-84. doi: 10.1002/stem.2060. Epub 2015 Jun 26. Stem Cells. 2015. PMID: 26013357 Free PMC article.
-
Effects of platelet-rich plasma on tooth replantation in dogs: a histologic and histomorphometric analysis.J Periodontal Implant Sci. 2018 Aug 31;48(4):224-235. doi: 10.5051/jpis.2018.48.4.224. eCollection 2018 Aug. J Periodontal Implant Sci. 2018. PMID: 30202606 Free PMC article.
-
Mesenchymal stem cell expression of SDF-1β synergizes with BMP-2 to augment cell-mediated healing of critical-sized mouse calvarial defects.J Tissue Eng Regen Med. 2017 Jun;11(6):1806-1819. doi: 10.1002/term.2078. Epub 2015 Jul 31. J Tissue Eng Regen Med. 2017. PMID: 26227988 Free PMC article.
-
Stem and progenitor cells: advancing bone tissue engineering.Drug Deliv Transl Res. 2016 Apr;6(2):159-73. doi: 10.1007/s13346-015-0235-1. Drug Deliv Transl Res. 2016. PMID: 25990836 Free PMC article. Review.
-
Characterization of Naturally Occurring Bioactive Factor Mixtures for Bone Regeneration.Int J Mol Sci. 2020 Feb 19;21(4):1412. doi: 10.3390/ijms21041412. Int J Mol Sci. 2020. PMID: 32093051 Free PMC article.
References
-
- Giannobile W.V. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S. - PubMed
-
- Chen F.M. Zhang M. Wu Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279. - PubMed
-
- Canalis E. Varghese S. McCarthy T.L. Centrella M. Role of platelet derived growth factor in bone cell function. Growth Regul. 1992;2:151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

