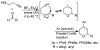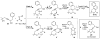Atom economical, one-pot, three-reaction cascade to novel tricyclic 2,4-dihydro-1H-benzo[f]isochromenes
- PMID: 23901903
- PMCID: PMC4447333
- DOI: 10.1021/ol401600j
Atom economical, one-pot, three-reaction cascade to novel tricyclic 2,4-dihydro-1H-benzo[f]isochromenes
Abstract
Reaction of 6-methyl-1-phenylhept-3-yne-2,6-diol with various aldehydes under Lewis acid conditions provides an atom economical, two-component cascade reaction sequence to novel 2,4-dihydro-1H-benzo[f]isochromene compounds. Aliphatic aldehydes as well as electron-deficient and -rich aromatic aldehydes can be used.
Figures
References
-
- Sheldon RA. Chem Soc Rev. 2012;41:1437–1451. - PubMed
- Trost BM. Handbook of Green Chemistry. 2012;7:1–33.
- Trost BM. Angew Chem Int Ed, Engl. 1995;34:259–281.
-
- Touré BB, Hall DG. Chem Rev. 2009;109:4439–4486. - PubMed
-
- Tietze L-F. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. - PubMed
-
-
For reactions described in the literature as cascade reactions, see: Horning BD, MacMillan DWC. J Am Chem Soc. 2013;135:6442–6445.Ohno H. Asian J Org Chem. 2013;2:18–28.Pintado-Sierra M, Rasero-Almansa AM, Corma A, Iglesias M, Sánchez F. J Cat. 2013;299:137–145.Surendra K, Qiu W, Corey EJ. J Am Chem Soc. 2011;133:9724–9726.Li H, Loh TP. Org Lett. 2010;12:2679–2681.Dalgard JE, Rychnovsky SD. Org Lett. 2005;7:1589–1591.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources