Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism
- PMID: 23902406
- PMCID: PMC3792005
- DOI: 10.1111/bph.12321
Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism
Abstract
Background and purpose: Epilepsy is the most prevalent neurological disease and is characterized by recurrent seizures. Here, we investigate (i) the anticonvulsant profiles of cannabis-derived botanical drug substances (BDSs) rich in cannabidivarin (CBDV) and containing cannabidiol (CBD) in acute in vivo seizure models and (ii) the binding of CBDV BDSs and their components at cannabinoid CB1 receptors.
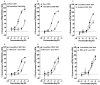
Experimental approach: The anticonvulsant profiles of two CBDV BDSs (50-422 mg·kg(-1) ) were evaluated in three animal models of acute seizure. Purified CBDV and CBD were also evaluated in an isobolographic study to evaluate potential pharmacological interactions. CBDV BDS effects on motor function were also investigated using static beam and grip strength assays. Binding of CBDV BDSs to cannabinoid CB1 receptors was evaluated using displacement binding assays.
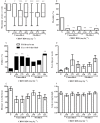
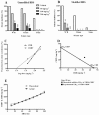
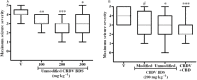
Key results: CBDV BDSs exerted significant anticonvulsant effects in the pentylenetetrazole (≥100 mg·kg(-1) ) and audiogenic seizure models (≥87 mg·kg(-1) ), and suppressed pilocarpine-induced convulsions (≥100 mg·kg(-1) ). The isobolographic study revealed that the anticonvulsant effects of purified CBDV and CBD were linearly additive when co-administered. Some motor effects of CBDV BDSs were observed on static beam performance; no effects on grip strength were found. The Δ(9) -tetrahydrocannabinol and Δ(9) -tetrahydrocannabivarin content of CBDV BDS accounted for its greater affinity for CB1 cannabinoid receptors than purified CBDV.
Conclusions and implications: CBDV BDSs exerted significant anticonvulsant effects in three models of seizure that were not mediated by the CB1 cannabinoid receptor and were of comparable efficacy with purified CBDV. These findings strongly support the further clinical development of CBDV BDSs for the treatment of epilepsy.
Keywords: anticonvulsant; cannabidiol; cannabidivarin; cannabinoid; epilepsy; isobologram; radioligand binding assays; seizure; tolerability.
© 2013 The British Pharmacological Society.
Figures

References
-
- BNF. British National Formulary. 62nd edn. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2011.
-
- Cheng YC, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. - PubMed
-
- Chesher G, Jackson D. Anticonvulsant effects of cannabinoids in mice: drug interactions within cannabinoids and cannabinoid interactions with phenytoin. Psychopharmacology (Berl) 1974;37:255–264. - PubMed
-
- Coenen A, Drinkenburg W, Inoue M, Van Luijtelaar E. Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res. 1992;12:75–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

