Evaluation of an In Silico PBPK Post-Bariatric Surgery Model through Simulating Oral Drug Bioavailability of Atorvastatin and Cyclosporine
- PMID: 23903405
- PMCID: PMC3697036
- DOI: 10.1038/psp.2013.23
Evaluation of an In Silico PBPK Post-Bariatric Surgery Model through Simulating Oral Drug Bioavailability of Atorvastatin and Cyclosporine
Abstract
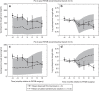
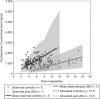
An increasing prevalence of morbid obesity has led to dramatic increases in the number of bariatric surgeries performed. Altered gastrointestinal physiology following surgery can be associated with modified oral drug bioavailability (Foral). In the absence of clinical data, an indication of changes to Foral via systems pharmacology models would be of value in adjusting dose levels after surgery. A previously developed virtual "post-bariatric surgery" population was evaluated through mimicking clinical investigations on cyclosporine and atorvastatin after bariatric surgery. Cyclosporine simulations displayed a reduced fraction absorbed through gut wall (fa) and Foral after surgery, consistent with reported observations. Simulated atorvastatin Foral postsurgery was broadly reflective of observed data with indications of counteracting interplay between reduced fa and an increased fraction escaping gut wall metabolism (FG). Inability to fully recover observed atorvastatin exposure after biliopancreatic diversion with duodenal switch highlights the current gap regarding the knowledge of associated biological changes.CPT: Pharmacometrics & Systems Pharmacology (2013) 2, e47; doi:10.1038/psp.2013.23; advance online publication 12 June 2013.
Figures



References
-
- Flegal K.M., Carroll M.D., Ogden C.L., Curtin L.R. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. - PubMed
-
- Organisation for Economics Co-Operation and Development (OECD) Obesity and the Economics of Prevention: Fit not Fat - United Kingdom (England) Key Facts . < http://www.oecd.org/document/58/0,3746,en_2649_33929 _46039034_1_1_1_1,0... > ( 2011Accessed December 2012
-
- Buchwald H., Oien D.M. Metabolic/bariatric surgery Worldwide 2008. Obes. Surg. 2009;19:1605–1611. - PubMed
-
- Picot J., et al. The clinical effectiveness and costeffectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol. Assess. 2009;13:215–357. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

