A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay
- PMID: 23903680
- PMCID: PMC3859338
- DOI: 10.1590/1414-431X20132647
A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay
Abstract
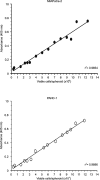
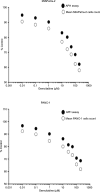
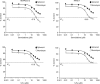
Current therapy for pancreatic cancer is multimodal, involving surgery and chemotherapy. However, development of pancreatic cancer therapies requires a thorough evaluation of drug efficacy in vitro before animal testing and subsequent clinical trials. Compared to two-dimensional culture of cell monolayer, three-dimensional (3-D) models more closely mimic native tissues, since the tumor microenvironment established in 3-D models often plays a significant role in cancer progression and cellular responses to the drugs. Accumulating evidence has highlighted the benefits of 3-D in vitro models of various cancers. In the present study, we have developed a spheroid-based, 3-D culture of pancreatic cancer cell lines MIAPaCa-2 and PANC-1 for pancreatic drug testing, using the acid phosphatase assay. Drug efficacy testing showed that spheroids had much higher drug resistance than monolayers. This model, which is characteristically reproducible and easy and offers rapid handling, is the preferred choice for filling the gap between monolayer cell cultures and in vivo models in the process of drug development and testing for pancreatic cancer.
Figures


References
-
- Sutherland RM, Durand RE. Radiation response of multicell spheroids - an in vitro tumour model. Curr Top Radiat Res Q. 1976;11:87–139. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

