Study of vaccinia and cowpox viruses' replication in Rac1-N17 dominant-negative cells
- PMID: 23903969
- PMCID: PMC3970603
- DOI: 10.1590/s0074-02762013000500004
Study of vaccinia and cowpox viruses' replication in Rac1-N17 dominant-negative cells
Abstract
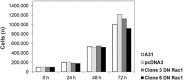
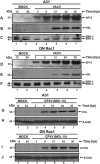
Interfering with cellular signal transduction pathways is a common strategy used by many viruses to create a propitious intracellular environment for an efficient replication. Our group has been studying cellular signalling pathways activated by the orthopoxviruses Vaccinia (VACV) and Cowpox (CPXV) and their significance to viral replication. In the present study our aim was to investigate whether the GTPase Rac1 was an upstream signal that led to the activation of MEK/ERK1/2, JNK1/2 or Akt pathways upon VACV or CPXV' infections. Therefore, we generated stable murine fibroblasts exhibiting negative dominance to Rac1-N17 to evaluate viral growth and the phosphorylation status of ERK1/2, JNK1/2 and Akt. Our results demonstrated that VACV replication, but not CPXV, was affected in dominant-negative (DN) Rac1-N17 cell lines in which viral yield was reduced in about 10-fold. Viral late gene expression, but not early, was also reduced. Furthermore, our data showed that Akt phosphorylation was diminished upon VACV infection in DN Rac1-N17 cells, suggesting that Rac1 participates in the phosphoinositide-3 kinase pathway leading to the activation of Akt. In conclusion, our results indicate that while Rac1 indeed plays a role in VACV biology, perhaps another GTPase may be involved in CPXV replication.
Figures




References
-
- Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–146. - PubMed
-
- Campos MAS, Kroon EG. Critical period for irreversible block of vaccinia virus replication. Rev Microbiol. 1993;24:104–110.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

