Cytoskeletal tropomyosins: choreographers of actin filament functional diversity
- PMID: 23904035
- PMCID: PMC3843815
- DOI: 10.1007/s10974-013-9355-8
Cytoskeletal tropomyosins: choreographers of actin filament functional diversity
Abstract
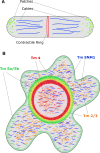
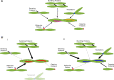
The actin cytoskeleton plays a central role in many essential cellular processes. Its involvement requires actin filaments to form multiple populations with different structural and therefore functional properties in specific subcellular locations. This diversity is facilitated through the interaction between actin and a number of actin binding proteins. One family of proteins, the tropomyosins, are absolutely essential in regulating actin's ability to form such diverse structures. In this review we integrate studies from different organisms and cell types in an attempt to provide a unifying view of tropomyosin dependent regulation of the actin cytoskeleton.
Figures

References
-
- Bryce NS, Schevzov G, Ferguson V, Percival JM, Lin JJ-C, Matsumura F, Bamburg JR, Jeffrey PL, Hardeman EC, Gunning P, Weinberger RP. Specification of actin filament function and molecular composition by tropomyosin isoforms. Mol Biol Cell. 2003;14(3):1002–1016. doi: 10.1091/mbc.E02-04-0244. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

