Day-surgery versus overnight stay surgery for laparoscopic cholecystectomy
- PMID: 23904112
- PMCID: PMC11491877
- DOI: 10.1002/14651858.CD006798.pub4
Day-surgery versus overnight stay surgery for laparoscopic cholecystectomy
Abstract
Background: Laparoscopic cholecystectomy is used to manage symptomatic gallstones. There is considerable controversy regarding whether it should be done as day-surgery or as an overnight stay surgery with regards to patient safety.
Objectives: To assess the impact of day-surgery versus overnight stay laparoscopic cholecystectomy on patient-oriented outcomes such as mortality, severe adverse events, and quality of life.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and mRCT until September 2012.
Selection criteria: We included randomised clinical trials comparing day-surgery versus overnight stay surgery for laparoscopic cholecystectomy, irrespective of language or publication status.
Data collection and analysis: Two authors independently assessed trials for inclusion and independently extracted the data. We analysed the data with both the fixed-effect and the random-effects models using Review Manager 5 analysis. We calculated the risk ratio (RR), mean difference (MD), or standardised mean difference (SMD) with 95% confidence intervals (CI) based on intention-to-treat or available case analysis.
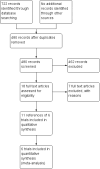
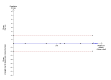
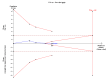
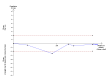
Main results: We identified a total of six trials at high risk of bias involving 492 participants undergoing day-case laparoscopic cholecystectomy (n = 239) versus overnight stay laparoscopic cholecystectomy (n = 253) for symptomatic gallstones. The number of participants in each trial ranged from 28 to 150. The proportion of women in the trials varied between 74% and 84%. The mean or median age in the trials varied between 40 and 47 years.With regards to primary outcomes, only one trial reported short-term mortality. However, the trial stated that there were no deaths in either of the groups. We inferred from the other outcomes that there was no short-term mortality in the remaining trials. Long-term mortality was not reported in any of the trials. There was no significant difference in the rate of serious adverse events between the two groups (4 trials; 391 participants; 7/191 (weighted rate 1.6%) in the day-surgery group versus 1/200 (0.5%) in the overnight stay surgery group; rate ratio 3.24; 95% CI 0.74 to 14.09). There was no significant difference in quality of life between the two groups (4 trials; 333 participants; SMD -0.11; 95% CI -0.33 to 0.10).There was no significant difference between the two groups regarding the secondary outcomes of our review: pain (3 trials; 175 participants; MD 0.02 cm visual analogue scale score; 95% CI -0.69 to 0.73); time to return to activity (2 trials, 217 participants; MD -0.55 days; 95% CI -2.18 to 1.08); and return to work (1 trial, 74 participants; MD -2.00 days; 95% CI -10.34 to 6.34). No significant difference was seen in hospital readmission rate (5 trials; 464 participants; 6/225 (weighted rate 0.5%) in the day-surgery group versus 5/239 (2.1%) in the overnight stay surgery group (rate ratio 1.25; 95% CI 0.43 to 3.63) or in the proportion of people requiring hospital readmissions (3 trials; 290 participants; 5/136 (weighted proportion 3.5%) in the day-surgery group versus 5/154 (3.2%) in the overnight stay surgery group; RR 1.09; 95% CI 0.33 to 3.60). No significant difference was seen in the proportion of failed discharge (failure to be discharged as planned) between the two groups (5 trials; 419 participants; 42/205 (weighted proportion 19.3%) in the day-surgery group versus 43/214 (20.1%) in the overnight stay surgery group; RR 0.96; 95% CI 0.65 to 1.41). For all outcomes except pain, the accrued information was far less than the diversity-adjusted required information size to exclude random errors.
Authors' conclusions: Day-surgery appears just as safe as overnight stay surgery in laparoscopic cholecystectomy. Day-surgery does not seem to result in improvement in any patient-oriented outcomes such as return to normal activity or earlier return to work. The randomised clinical trials backing these statements are weakened by risks of systematic errors (bias) and risks of random errors (play of chance). More randomised clinical trials are needed to assess the impact of day-surgery laparoscopic cholecystectomy on the quality of life as well as other outcomes of patients.
Conflict of interest statement
None known.
Figures
























Update of
-
Day-case versus overnight stay for laparoscopic cholecystectomy.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD006798. doi: 10.1002/14651858.CD006798.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2013 Jul 31;(7):CD006798. doi: 10.1002/14651858.CD006798.pub4. PMID: 18677781 Updated.
References
References to studies included in this review
Barthelsson 2008 {published data only}
-
- Barthelsson C, Anderberg B, Ramel S, Bjorvell C, Giesecke K, Nordstrom G. Outpatient versus inpatient laparoscopic cholecystectomy: A prospective randomized study of symptom occurrence, symptom distress and general state of health during the first post‐operative week. Journal of Evaluation in Clinical Practice 2008;14(4):577‐84. - PubMed
Dirksen 2001 {published data only}
-
- Dirksen CD, Schmitz RF, Hans KM, Nieman FHM, Hoogenboom LJ, Go PMNYH. Laparoscopic cholecystectomy in an ambulatory treatment is just as effective as an overnight stay and from a social perspective is cheaper; a randomised study. Nederlands Tijdschrift voor Geneeskunde 2001;145(50):2434‐9. - PubMed
Hollington 1999 {published data only}
-
- Hollington P, Toogood GJ, Padbury RT. A prospective randomized trial of day‐stay only versus overnight‐stay laparoscopic cholecystectomy. Australia and New Zealand Journal of Surgery 1999;69(12):841‐3. - PubMed
Johansson 2006 {published and unpublished data}
-
- Johansson M, Thune A, Nelvin L, Lundell L. Randomized clinical trial of day‐care versus overnight‐stay laparoscopic cholecystectomy. British Journal of Surgery 2006;93(1):40‐5. - PubMed
-
- Thune A, Nelvin L, Johansson MG, Lundell L. Randomized clinical trial of day‐care versus overnight stay laparoscopic cholecystectomy. Gastroenterology 2005;128(4):A785. - PubMed
Keulemans 1998 {published data only}
-
- Keulemans YCA, Eshuis JH, Haes J, Leeuwenberg A, Wit TL, Gouma DJ. Day care or hospital admission after laparoscopic cholecystectomy, a prospective randomized trial. Gastroenterology 1998;114(4):A1271.
-
- Keulemans YCA, Eshuis JH, Haes JCJM. Laparoscopic cholecystectomy: admittance or day care? A randomised study. Nederlands Tijdschrift voor Geneeskunde 1998;142(14):822.
-
- Keulemans YCA, Eshuis JH, Haes JCJM, Wit L, Gouma DJ. Laparoscopic cholecystectomy in day care as effective as during hospitalization, and cheaper; a randomized trial. Nederlands Tijdschrift voor Geneeskunde 1999;143(12):621‐6.
-
- Keulemans YCA, Eshuis JH, Haes JCJM, Leeuwenberg A, Wit L, Gouma DJ. Day care or hospital admission after laparoscopic cholecystectomy, a prospective randomized trial [abstract]. European Journal of Gastroenterology & Hepatology 1998;10(Suppl 12):A25.
Young 2001 {published and unpublished data}
-
- Young J, O'Connell B. Recovery following laparoscopic cholecystectomy in either a 23 hour or an 8 hour facility. Journal of Quality in Clinical Practice 2001;21(1‐2):2‐7. - PubMed
References to studies excluded from this review
Bews‐Hair 2000 {published data only}
-
- Bews‐Hair M, Coulter G, Frizelle FA. A prospective randomized trial of day‐stay only versus overnight‐stay laparoscopic cholecystectomy: comment [1]. Australian and New Zealand Journal of Surgery 2000;70(10):743. - PubMed
Burney 2002 {published data only}
-
- Burney RE, Jones KR. Ambulatory and admitted laparoscopic cholecystectomy patients have comparable outcomes but different functional health status. Surgical Endoscopy 2002;16(6):921‐6. - PubMed
Curet 2002 {published and unpublished data}
-
- Curet MJ, Contreras M, Weber DM, Albrecht R. Laparoscopic cholecystectomy ‐ outpatient vs inpatient management. Surgical Endoscopy 2002;16(3):453‐7. - PubMed
Parvaiz 2006 {published data only}
-
- Parvaiz MA, Hafeez R. Randomized clinical trial of day‐care versus overnight‐stay laparoscopic cholecystectomy (Br J Surg 2006; 93: 40‐5). British Journal of Surgery 2006;93(5):639‐40. - PubMed
Rosen 2001 {published data only}
-
- Rosen MJ, Malm JA, Tarnoff M, Zuccala K, Ponsky JL. Cost‐effectiveness of ambulatory laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 2001;11(3):182‐4. - PubMed
Selas 2004 {published data only}
-
- Selas PR, Santiuste AC. Laparoscopic cholecystectomy and outpatient surgery. Revista Espanola de Enfermedades Digestivas 2004;96(7):435‐8. - PubMed
Sharma 2004 {published data only}
-
- Sharma A, Hayden JD, Reese RA, Sedman PC, Royston CMS, O’Boyle CJ. Prospective comparison of ambulatory with inpatient laparoscopic cholecystectomy: Outcome, patient preference and satisfaction. Ambulatory Surgery 2004;11(1‐2):23‐6.
Additional references
AAGBI 2011
-
- AAGBI. Day case and short stay surgery: 2. Anaesthesia 2011;66(5):417‐34. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ 2011 (accessed 5 July 2013).
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Egger 1997
Giger 2011
-
- Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. The British Journal of Surgery 2011;98(3):391‐6. - PubMed
Gluud 2012
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 1. Art. No.: LIVER.
GradePro 3.6 [Computer program]
-
- Brozek JL, Oxman A, Schünemann HJ. Grade Profiler 3.6. Version 3.6. Grade Profiler, 2004‐2007.
Gurusamy 2009
-
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. - PubMed
Halldestam 2004
-
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. - PubMed
HES 2012
-
- Hospital Episode Statistics. Main operations. 4 character. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&c... (accessed 5 July 2013).
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Jørgensen 1987
-
- Jørgensen T. Prevalence of gallstones in a Danish population. American Journal of Epidemiology 1987;126(5):912‐21. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Muhrbeck 1995
-
- Muhrbeck O, Ahlberg J. Prevalence of gallstone disease in a Swedish population. Scandinavian Journal of Gastroenterology 1995;30(11):1125‐8. - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
NIH 1992
-
- NIH. Gallstones and Laparoscopic Cholecystectomy, NIH Consens Statement Online 1992 Sep 14‐16. http://consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm 1992 (accessed 28 February 2007); Vol. 10, issue 3:1‐20. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Shamiyeh 2004
-
- Shamiyeh A, Wayand W. Laparoscopic cholecystectomy: early and late complications and their treatment. Langenbecks Archives of Surgery 2004;389(3):164‐71. - PubMed
SPIRIT 2013
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 5 July 2013).
Todd 1996
-
- Todd KH, Funk JP. The minimum clinically important difference in physician‐assigned visual analog pain scores. Academic Emergency Medicine 1996;3(2):142‐6. [PUBMED: 8808375] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
References to other published versions of this review
Gurusamy 2008a
Gurusamy 2008b
Gurusamy 2008c
-
- Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta‐analysis of randomized controlled trials on the safety and effectiveness of day‐case laparoscopic cholecystectomy. British Journal of Surgery 2008;95(2):161‐8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

