Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration
- PMID: 23904157
- PMCID: PMC3748335
- DOI: 10.4049/jimmunol.1203421
Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration
Abstract
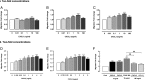
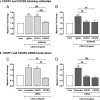
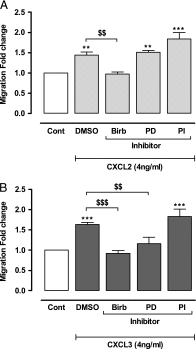
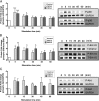
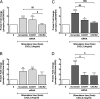
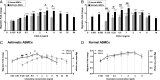
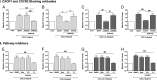
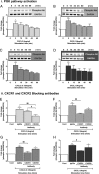
Structural cell migration plays a central role in the pathophysiology of several diseases, including asthma. Previously, we established that IL-17-induced (CXCL1, CXCL2, and CXCL3) production promoted airway smooth muscle cell (ASMC) migration, and consequently we sought to investigate the molecular mechanism of CXC-induced ASMC migration. Recombinant human CXCL1, CXCL2, and CXCL3 were used to assess migration of human primary ASMCs from normal and asthmatic subjects using a modified Boyden chamber. Neutralizing Abs or small interfering RNA (siRNA) knockdown and pharmacological inhibitors of PI3K, ERK1/2, and p38 MAPK pathways were used to investigate the receptors and the signaling pathways involved in CXC-induced ASMC migration, respectively. We established the ability of CXCL2 and CXCL3, but not CXCL1, to induce ASMC migration at the tested concentrations using normal ASMCs. We found CXCL2-induced ASMC migration to be dependent on p38 MAPK and CXCR2, whereas CXCL3-induced migration was dependent on p38 and ERK1/2 MAPK pathways via CXCR1 and CXCR2. While investigating the effect of CXCL2 and CXCL3 on asthmatic ASMC migration, we found that they induced greater migration of asthmatic ASMCs compared with normal ones. Interestingly, unlike normal ASMCs, CXCL2- and CXCL3-induced asthmatic ASMC migration was mainly mediated by the PI3K pathway through CXCR1. In conclusion, our results establish a new role of CXCR1 in ASMC migration and demonstrate the diverse mechanisms by which CXCL2 and CXCL3 mediate normal and asthmatic ASMC migration, suggesting that they may play a role in the pathogenesis of airway remodeling in asthma.
Figures
References
-
- Ramos-Barbón D., Fraga-Iriso R., Brienza N. S., Montero-Martínez C., Verea-Hernando H., Olivenstein R., Lemiere C., Ernst P., Hamid Q. A., Martin J. G. 2010. T cells localize with proliferating smooth muscle α-actin+ cell compartments in asthma. Am. J. Respir. Crit. Care Med. 182: 317–324 - PubMed
-
- Takeda N., Sumi Y., Préfontaine D., Al Abri J., Al Heialy N., Al-Ramli W., Michoud M. C., Martin J. G., Hamid Q. 2009. Epithelium-derived chemokines induce airway smooth muscle cell migration. Clin. Exp. Allergy 39: 1018–1026 - PubMed
-
- Kaur D., Saunders R., Berger P., Siddiqui S., Woodman L., Wardlaw A., Bradding P., Brightling C. E. 2006. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am. J. Respir. Crit. Care Med. 174: 1179–1188 - PubMed
-
- Chang Y., Al-Alwan L., Risse P. A., Roussel L., Rousseau S., Halayko A. J., Martin J. G., Hamid Q., Eidelman D. H. 2011. TH17 cytokines induce human airway smooth muscle cell migration. J. Allergy Clin. Immunol. 127: 1046–1053.e2 - PubMed
-
- Parameswaran K., Radford K., Fanat A., Stephen J., Bonnans C., Levy B. D., Janssen L. J., Cox P. G. 2007. Modulation of human airway smooth muscle migration by lipid mediators and Th-2 cytokines. Am. J. Respir. Cell Mol. Biol. 37: 240–247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

