Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury
- PMID: 23904166
- PMCID: PMC3777242
- DOI: 10.4049/jimmunol.1202733
Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury
Abstract
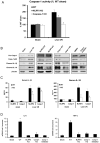
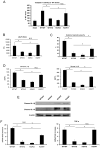
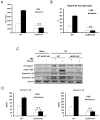
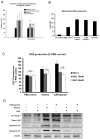
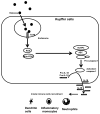
Cellular processes that drive sterile inflammatory injury after hepatic ischemia/reperfusion (I/R) injury are not completely understood. Activation of the inflammasome plays a key role in response to invading intracellular pathogens, but mounting evidence suggests that it also plays a role in inflammation driven by endogenous danger-associate molecular pattern molecules released after ischemic injury. The nucleotide-binding domain, leucine-rich repeat containing protein 3 (NLRP3) inflammasome is one such process, and the mechanism by which its activation results in damage and inflammatory responses following liver I/R is unknown. In this article, we report that both NLRP3 and its downstream target caspase-1 are activated during I/R and are essential for hepatic I/R injury, because both NLRP3 and caspase-1 knockout mice are protected from injury. Furthermore, inflammasome-mediated injury is dependent on caspase-1 expression in liver nonparenchymal cells. Although upstream signals that activate the inflammasome during ischemic injury are not well characterized, we show that endogenous extracellular histones activate the NLRP3 inflammasome during liver I/R through TLR9. This occurs through TLR9-dependent generation of reactive oxygen species. This mechanism is operant in resident liver Kupffer cells, which drive innate immune responses after I/R injury by recruiting additional cell types, including neutrophils and inflammatory monocytes. These novel findings illustrate a new mechanism by which extracellular histones and activation of NLRP3 inflammasome contribute to liver damage and the activation of innate immunity during sterile inflammation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

