Functional activity of RLIM/Rnf12 is regulated by phosphorylation-dependent nucleocytoplasmic shuttling
- PMID: 23904271
- PMCID: PMC3784382
- DOI: 10.1091/mbc.E13-05-0239
Functional activity of RLIM/Rnf12 is regulated by phosphorylation-dependent nucleocytoplasmic shuttling
Abstract
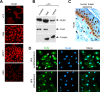
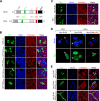
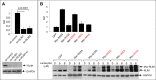
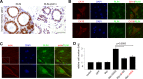
The X-linked gene Rnf12 encodes the ubiquitin ligase really interesting new gene (RING) finger LIM domain-interacting protein (RLIM)/RING finger protein 12 (Rnf12), which serves as a major sex-specific epigenetic regulator of female mouse nurturing tissues. Early during embryogenesis, RLIM/Rnf12 expressed from the maternal allele is crucial for the development of extraembryonic trophoblast cells. In contrast, in mammary glands of pregnant and lactating adult females RLIM/Rnf12 expressed from the paternal allele functions as a critical survival factor for milk-producing alveolar cells. Although RLIM/Rnf12 is detected mostly in the nucleus, little is known about how and in which cellular compartment(s) RLIM/Rnf12 mediates its biological functions. Here we demonstrate that RLIM/Rnf12 protein shuttles between nucleus and cytoplasm and this is regulated by phosphorylation of serine S214 located within its nuclear localization sequence. We show that shuttling is important for RLIM to exert its biological functions, as alveolar cell survival activity is inhibited in cells expressing shuttling-deficient nuclear or cytoplasmic RLIM/Rnf12. Thus regulated nucleocytoplasmic shuttling of RLIM/Rnf12 coordinates cellular compartments during mammary alveolar cell survival.
Figures



References
-
- Bach I. The LIM domain: regulation by association. Mech Dev. 2000;91:5–17. - PubMed
-
- Bach I, Carriere C, Ostendorff HP, Andersen B, Rosenfeld MG. A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev. 1997;11:1370–1380. - PubMed
-
- Bach I, Ostendorff HP. Orchestrating nuclear functions: ubiquitin sets the rhythm. Trends Biochem Sci. 2003;28:189–195. - PubMed
-
- Bach I, Rodriguez-Esteban C, Carriere C, Bhushan A, Krones A, Rose DW, Glass CK, Andersen B, Izpisua Belmonte JC, Rosenfeld MG. RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex. Nat Genet. 1999;22:394–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

