A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study)
- PMID: 23904473
- PMCID: PMC3888614
- DOI: 10.1136/annrheumdis-2013-203523
A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study)
Abstract
Objectives: This study compared the efficacy and safety of subcutaneous (SC) versus intravenous (IV) formulations of tocilizumab in patients with rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs (DMARD).
Methods: Patients (n=1262) were randomly assigned to receive tocilizumab-SC 162 mg weekly+placebo-IV every 4 weeks or tocilizumab-IV 8 mg/kg every 4 weeks+placebo-SC weekly in combination with traditional DMARD. The primary outcome was to demonstrate the non-inferiority of tocilizumab-SC to tocilizumab-IV with regard to the proportion of patients in each group achieving an American College of Rheumatology (ACR) 20 response at week 24 using a 12% non-inferiority margin (NIM). Secondary outcomes were disease activity score using 28 joints (DAS28), ACR responses, health assessment questionnaire scores and safety assessments.
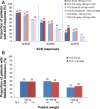
Results: At week 24, 69.4% (95% CI 65.5 to 73.2) of tocilizumab-SC-treated patients versus 73.4% (95% CI 69.6 to 77.1) of tocilizumab-IV-treated patients achieved an ACR20 response (weighted difference between groups -4.0%, 95% CI -9.2 to 1.2); the 12% NIM was met. ACR50/70 responses, DAS28 and physical function improvements were comparable between the tocilizumab-SC and tocilizumab-IV groups. The safety profiles of tocilizumab-SC and tocilizumab-IV were similar, and the most common adverse event was infection. Injection-site reactions (ISR) occurred more frequently in the tocilizumab-SC group than in the tocilizumab-IV (placebo-SC) group. No anaphylaxis was reported over the 24 weeks.
Conclusions: Tocilizumab-SC 162 mg weekly demonstrated comparable efficacy to tocilizumab-IV 8 mg/kg. The safety profile of tocilizumab-SC is consistent with the known and well-established safety profile of tocilizumab-IV, with the exception of a higher incidence of ISR, which were more common with tocilizumab-SC administration.
Keywords: DMARDs (biologic); Disease Activity; Rheumatoid Arthritis.
Figures


References
-
- Scott DL. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther 2012;91:30–43 - PubMed
-
- Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29 - PubMed
-
- Ohta S, Tsuru T, Terao K. Optimal dose prediction by pharmacokinetic and biomarker response of subcutaneous tocilizumab treatment a Phase I/II study evaluating the safety, pharmacokinetics and clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2010;62:S1115
-
- Stubenrauch K, Wessels U, Birnboeck H, et al. Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: Evaluation of an antidrug antibody ELISA using clinical adverse event-driven immunogenicity testing. Clin Ther 2010;32:1597–609 - PubMed
-
- Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

