Structural and functional changes across the visual cortex of a patient with visual form agnosia
- PMID: 23904613
- PMCID: PMC6618540
- DOI: 10.1523/JNEUROSCI.4853-12.2013
Structural and functional changes across the visual cortex of a patient with visual form agnosia
Abstract
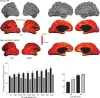
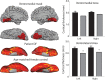
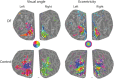
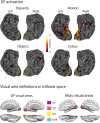
Loss of shape recognition in visual-form agnosia occurs without equivalent losses in the use of vision to guide actions, providing support for the hypothesis of two visual systems (for "perception" and "action"). The human individual DF received a toxic exposure to carbon monoxide some years ago, which resulted in a persisting visual-form agnosia that has been extensively characterized at the behavioral level. We conducted a detailed high-resolution MRI study of DF's cortex, combining structural and functional measurements. We present the first accurate quantification of the changes in thickness across DF's occipital cortex, finding the most substantial loss in the lateral occipital cortex (LOC). There are reduced white matter connections between LOC and other areas. Functional measures show pockets of activity that survive within structurally damaged areas. The topographic mapping of visual areas showed that ordered retinotopic maps were evident for DF in the ventral portions of visual cortical areas V1, V2, V3, and hV4. Although V1 shows evidence of topographic order in its dorsal portion, such maps could not be found in the dorsal parts of V2 and V3. We conclude that it is not possible to understand fully the deficits in object perception in visual-form agnosia without the exploitation of both structural and functional measurements. Our results also highlight for DF the cortical routes through which visual information is able to pass to support her well-documented abilities to use visual information to guide actions.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
