Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury
- PMID: 23904622
- PMCID: PMC3728693
- DOI: 10.1523/JNEUROSCI.2121-13.2013
Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury
Abstract
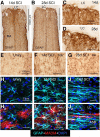
Astroglial scars surround damaged tissue after trauma, stroke, infection, or autoimmune inflammation in the CNS. They are essential for wound repair, but also interfere with axonal regrowth. A better understanding of the cellular mechanisms, regulation, and functions of astroglial scar formation is fundamental to developing safe interventions for many CNS disorders. We used wild-type and transgenic mice to quantify and dissect these parameters. Adjacent to crush spinal cord injury (SCI), reactive astrocytes exhibited heterogeneous phenotypes as regards proliferation, morphology, and chemistry, which all varied with distance from lesions. Mature scar borders at 14 d after SCI consisted primarily of newly proliferated astroglia with elongated cell processes that surrounded large and small clusters of inflammatory, fibrotic, and other cells. During scar formation from 5 to 14 d after SCI, cell processes deriving from different astroglia associated into overlapping bundles that quantifiably reoriented and organized into dense mesh-like arrangements. Selective deletion of STAT3 from astroglia quantifiably disrupted the organization of elongated astroglia into scar borders, and caused a failure of astroglia to surround inflammatory cells, resulting in increased spread of these cells and neuronal loss. In cocultures, wild-type astroglia spontaneously corralled inflammatory or fibromeningeal cells into segregated clusters, whereas STAT3-deficient astroglia failed to do so. These findings demonstrate heterogeneity of reactive astroglia and show that scar borders are formed by newly proliferated, elongated astroglia, which organize via STAT3-dependent mechanisms to corral inflammatory and fibrotic cells into discrete areas separated from adjacent tissue that contains viable neurons.
Conflict of interest statement
The authors have no financial conflicts or interests.
Figures











References
-
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
