An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy
- PMID: 23905526
- PMCID: PMC3778735
- DOI: 10.1164/rccm.201305-0923ST
An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy
Abstract
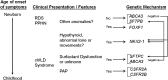
Background: There is growing recognition and understanding of the entities that cause interstitial lung disease (ILD) in infants. These entities are distinct from those that cause ILD in older children and adults.
Methods: A multidisciplinary panel was convened to develop evidence-based guidelines on the classification, diagnosis, and management of ILD in children, focusing on neonates and infants under 2 years of age. Recommendations were formulated using a systematic approach. Outcomes considered important included the accuracy of the diagnostic evaluation, complications of delayed or incorrect diagnosis, psychosocial complications affecting the patient's or family's quality of life, and death.
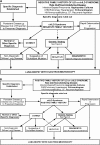
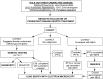
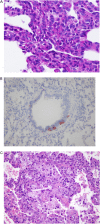
Results: No controlled clinical trials were identified. Therefore, observational evidence and clinical experience informed judgments. These guidelines: (1) describe the clinical characteristics of neonates and infants (<2 yr of age) with diffuse lung disease (DLD); (2) list the common causes of DLD that should be eliminated during the evaluation of neonates and infants with DLD; (3) recommend methods for further clinical investigation of the remaining infants, who are regarded as having "childhood ILD syndrome"; (4) describe a new pathologic classification scheme of DLD in infants; (5) outline supportive and continuing care; and (6) suggest areas for future research.
Conclusions: After common causes of DLD are excluded, neonates and infants with childhood ILD syndrome should be evaluated by a knowledgeable subspecialist. The evaluation may include echocardiography, controlled ventilation high-resolution computed tomography, infant pulmonary function testing, bronchoscopy with bronchoalveolar lavage, genetic testing, and/or lung biopsy. Preventive care, family education, and support are essential.
Figures


References
-
- Bokulic RE, Hilman BC. Interstitial lung disease in children. Pediatr Clin North Am. 1994;41:543–567. - PubMed
-
- Fan LL, Langston C. Chronic interstitial lung disease in children. Pediatr Pulmonol. 1993;16:184–196. - PubMed
-
- Nicholson AG, Kim H, Corrin B, Bush A, du Bois RM, Rosenthal M, Sheppard MN. The value of classifying interstitial pneumonitis in childhood according to defined histological patterns. Histopathology. 1998;33:203–211. - PubMed
-
- Deutsch GH, Albright E, Chou PM, Cool CD, Coventry S, Davis MM, Dishop MK, Galambos C, Patterson K, Wert SE, et al. Defining the spectrum of diffuse lung disease in infancy: a working classification of the pediatric interstitial lung disease cooperative [abstract] Mod Pathol. 2005;18:304.
-
- Langston C, Dishop M. Infant lung biopsy: clarifying the pathologic spectrum. Pathol Int. 2004;54:S419–S421.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

