Platelets, coagulation and fibrinolysis in breast cancer progression
- PMID: 23905544
- PMCID: PMC3979075
- DOI: 10.1186/bcr3425
Platelets, coagulation and fibrinolysis in breast cancer progression
Abstract
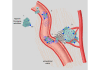
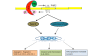
The progression of breast cancer from early-stage to metastatic disease results from a series of events during which malignant cells invade and travel within the bloodstream to distant sites, leading to a clonogenic accumulation of tumor cells in non-breast tissue. While mechanistically complex, an emerging literature supports hemostatic elements as an important patient factor that facilitates the metastatic potential of breast cancer. Hemostatic elements involved include platelets, coagulation, and fibrinolysis. Key steps in breast tumor progression, including cellular transformation, proliferation, tumor cell survival, and angiogenesis, can be mediated by components of the hemostatic system. Thus, the hemostatic system provides potential targets for novel therapeutic approaches to breast cancer therapy with drugs in current use and in development. The present article provides a comprehensive overview of the evidence and mechanisms supporting the roles played by platelets, coagulation activation, and the fibrinolytic system in breast cancer progression.
Figures

References
-
- Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;15:70–76. - PubMed
-
- Caine GJ, Lip GY, Stonelake PS, Ryan P, Blann AD. Platelet activation, coagulation and angiogenesis in breast and prostate carcinoma. Thromb Haemost. 2004;15:185–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

