Characterization of the late endosomal ESCRT machinery in Trypanosoma brucei
- PMID: 23905922
- PMCID: PMC3806108
- DOI: 10.1111/tra.12094
Characterization of the late endosomal ESCRT machinery in Trypanosoma brucei
Abstract
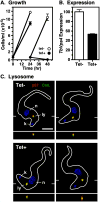
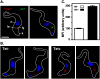
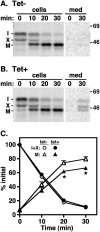
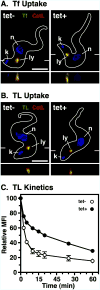
The multivesicular body (MVB) is a specialized Rab7+ late endosome (LE) containing multiple intralumenal vesicles that function in targeting ubiquitinylated cell surface proteins to the lysosome for degradation. African trypanosomes lack a morphologically well-defined MVB, but contain orthologs of the ESCRT (Endosomal Sorting Complex Required for Transport) machinery that mediates MVB formation. We investigate the role of TbVps23, an early ESCRT component, and TbVps4, the terminal ESCRT ATPase, in lysosomal trafficking in bloodstream form trypanosomes. Both localize to the TbRab7+ LE and RNAi silencing of each rapidly blocks growth. TbVps4 silencing results in approximately threefold accumulation of TbVps23 at the LE, consistent with blocking terminal ESCRT disassembly. Trafficking of endocytic and biosynthetic cargo, but not default lysosomal reporters, is also negatively affected. Others reported that TbVps23 mediates ubiquitin-dependent lysosomal degradation of invariant surface glycoproteins (ISG65) (Leung et al., Traffic 2008;9:1698-1716). In contrast, we find that TbVps23 ablation does not affect ISG65 turnover, while TbVps4 silencing markedly enhances lysosomal degradation. We propose several models to accommodate these results, including that the ESCRT machinery actually retrieves ISG65 from the LE to earlier endocytic compartments, and in its absence ISG65 traffics more efficiently to the lysosome. Overall, these results confirm that the ESCRT machinery is essential in Trypanosoma brucei and plays important and novel role(s) in LE function in trypanosomes.
Keywords: ESCRT; MVB; invariant surface glycoprotein; late endosome; lysosome; trypanosome.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicting interests to report.
Figures





References
-
- Rudenko G. African trypanosomes: the genome and adaptations for immune evasion. Essays Biochem. 2011;51:47–62. - PubMed
-
- Schwede A, Carrington M. Bloodstream form trypanosome plasma membrane proteins: antigenic variation and invariant antigens. Parasitol. 2010;137:2029–2039. - PubMed
-
- Langreth SG, Balber AE. Protein uptake and digestion in bloodstream and culture forms of Trypanosoma brucei. J Protozool. 1975;22:40–53. - PubMed
-
- Morgan GW, Allen CL, Jeffries TR, Hollinshead M, Field MC. Developmental and morphological regulation of clathrin-mediated endocytosis in Trypanosoma brucei. J Cell Sci. 2001;114:2605–2015. - PubMed
-
- Pamer EG, So M, Davis CE. Identification of a developmentally regulated cysteine protease of Trypanosoma brucei. Mol Biochem Parasitol. 1989;33:27–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

