Cyclopropenimine-catalyzed enantioselective Mannich reactions of tert-butyl glycinates with N-Boc-imines
- PMID: 23906087
- PMCID: PMC3804837
- DOI: 10.1021/ja407277a
Cyclopropenimine-catalyzed enantioselective Mannich reactions of tert-butyl glycinates with N-Boc-imines
Abstract
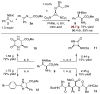
Cyclopropenimine 1 is shown to catalyze Mannich reactions between glycine imines and N-Boc-aldimines with high levels of enantio- and diastereocontrol. The reactivity of 1 is shown to be substantially greater than that of a widely used thiourea cinchona alkaloid-derived catalyst. A variety of aryl and aliphatic N-Boc-aldimines are effective substrates for this transformation. A preparative-scale reaction to deliver >90 mmol of product is shown using 1 mol % catalyst. The products of this transformation can be converted into several useful derivatives.
Figures


References
-
-
For a review, see: Gómez Arrayás R, Carretero JC. Chem Soc Rev. 2009;38:1940.
-
-
- Bandar JS, Lambert TH. J Am Chem Soc. 2012;134:5552. - PubMed
-
-
See supporting information for an improved synthesis of cyclopropenimine 1 directly from pentachlorocyclopropane.
-
-
-
(S)-2-(2,3-bis(dicyclohexylamino)cyclopropenimine)-3-phenylpropan-1-ol hydrochloride (1•HCl) can be purchased from Sigma-Aldrich (product #L511188).
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

