UPR-inducible miRNAs contribute to stressful situations
- PMID: 23906563
- PMCID: PMC4056666
- DOI: 10.1016/j.tibs.2013.06.012
UPR-inducible miRNAs contribute to stressful situations
Abstract
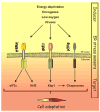
The endoplasmic reticulum (ER) senses both extracellular and intracellular stresses that can disrupt its ability to facilitate the maturation of proteins destined for secretory pathways. The accumulation of misfolded proteins within the ER triggers an adaptive signaling pathway coined the unfolded protein response (UPR). UPR activation contributes to cell adaptation by reducing the rate of protein translation while increasing the synthesis of chaperones. Although we have gained considerable insight into the mechanisms that regulate gene expression and certain aspects of protein translation, the contribution of miRNAs to UPR-dependent activities has only recently been investigated. Here we highlight recent insights into the contribution of miRNAs to UPR-dependent cellular adaptive responses.
Keywords: ER stress; activating transcription factor 6 (ATF6); apoptosis; inositol-regulated enzyme 1α (IRE1α); microRNA; protein kinase RNA-like endoplasmic reticulum kinase (PERK); unfolded protein response (UPR).
2013 Elsevier Ltd. All rights reserved
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

