Prevalence of hematopoietic cell transplant survivors in the United States
- PMID: 23906634
- PMCID: PMC3779514
- DOI: 10.1016/j.bbmt.2013.07.020
Prevalence of hematopoietic cell transplant survivors in the United States
Abstract
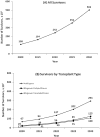
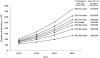
Advances in hematopoietic cell transplantation (HCT) have led to an increasing number of transplant survivors. To adequately support their healthcare needs, there is a need to know the prevalence of HCT survivors. We used data on 170,628 recipients of autologous and allogeneic HCT reported to the Center for International Blood and Marrow Transplant Research from 1968 to 2009 to estimate the current and future number of HCT survivors in the United States. Stacked cohort simulation models were used to estimate the number of HCT survivors in the United States in 2009 and to make projections for HCT survivors by the year 2030. There were 108,900 (range, 100,500 to 115,200) HCT survivors in the United States in 2009. This included 67,000 autologous HCT and 41,900 allogeneic HCT survivors. The number of HCT survivors is estimated to increase by 2.5 times by the year 2020 (242,000 survivors) and 5 times by the year 2030 (502,000 survivors). By 2030, the age at transplant will be < 18 years for 14% of all survivors (n = 64,000), 18 to 59 years for 61% survivors (n = 276,000), and 60 years and older for 25% of survivors (n = 113,000). In coming decades, a large number of individuals will be HCT survivors. Transplant center providers, hematologists, oncologists, primary care physicians, and other specialty providers will need to be familiar with the unique and complex health issues faced by this population.
Keywords: Allogeneic; Autologous; Hematopoietic cell transplantation; Prevalence; Survivors.
Copyright © 2013 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors has any relevant financial conflicts of interest to disclose. Navneet Majhail had full access to all of the data in the study and had final responsibility for the integrity of the data, the accuracy of the data analysis, and the responsibility for the decision to submit for publication. Authors designed research (all authors), collected data (NM), performed statistical analysis (LT, KK), interpreted data (all authors), drafted the manuscript (NM, LT), and critically revised the manuscript (all authors). All authors have reviewed and approved the final version of the manuscript.
Figures

References
-
- Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2007. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010 (accessed 01/01/2013)
-
- Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2011 Available at: http://www.cibmtr.org.
-
- Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29(7):805–813. doi: 10.1200/JCO.2010.32.5001. Prepublished on 2011/01/12 as. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

