Independent radiologic review of the Gynecologic Oncology Group Study 0218, a phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer
- PMID: 23906656
- PMCID: PMC3795907
- DOI: 10.1016/j.ygyno.2013.07.100
Independent radiologic review of the Gynecologic Oncology Group Study 0218, a phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer
Abstract
Objectives: Gynecologic Oncology Group Study 0218 (GOG-0218), a phase III, placebo-controlled trial in newly diagnosed stage III/IV ovarian cancer (OC), demonstrated a benefit in investigator (INV)-assessed progression-free survival (PFS) with bevacizumab (BEV) administered with and following carboplatin/paclitaxel (CP) for up to 15 months vs. CP alone. To determine the reliability of Response Evaluation Criteria in Solid Tumors (RECIST) in assessing disease progression (PD) in GOG-0218, an independent review of radiologic and clinical data (IRC) was conducted.
Methods: Blinded reviews followed RECIST 1.0 in accordance with the study protocol; PFS was analyzed in the intent-to-treat population.
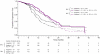
Results: CP+BEV→BEV achieved a significant PFS improvement in both assessments. Hazard ratios for PFS (IRC: 0.623; 95% confidence interval [CI]: 0.503-0.772; p<0.0001 vs. INV: 0.624; 95% CI: 0.520-0.749; p<0.0001) and the improvement in median PFS (IRC: 19.1 and 13.1 months vs. INV: 18.2 and 12 months) were similar between IRC and INV assessments. There was high concordance between IRC- and INV-determined PD status (77%) and date (73%). Subgroup analyses were consistent with the primary IRC findings. Early and late discontinuation discordance measures showed no evidence of INV bias.
Conclusion: IRC analysis confirmed a significant PFS improvement with CP+BEV→BEV vs. CP alone. Concordance was not influenced by extent of residual disease after cytoreductive surgery or initial stage. The IRC size, high participation rate, and strong concordance between IRC and INV assessments suggest that RECIST can be applied objectively in OC studies.
Keywords: Bevacizumab; IRC; Independent review; Ovarian cancer; Phase 3.
© 2013.
Figures

Similar articles
-
Independent radiologic review of AURELIA, a phase 3 trial of bevacizumab plus chemotherapy for platinum-resistant recurrent ovarian cancer.Gynecol Oncol. 2016 Sep;142(3):465-70. doi: 10.1016/j.ygyno.2016.05.011. Epub 2016 Jun 25. Gynecol Oncol. 2016. PMID: 27184721 Clinical Trial.
-
Progression-free survival by local investigator versus independent central review: comparative analysis of the AGO-OVAR16 Trial.Gynecol Oncol. 2015 Jan;136(1):37-42. doi: 10.1016/j.ygyno.2014.11.074. Epub 2014 Nov 28. Gynecol Oncol. 2015. PMID: 25434635 Clinical Trial.
-
First-line bevacizumab plus chemotherapy in Chinese patients with stage III/IV epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer: a phase III randomized controlled trial.J Gynecol Oncol. 2024 Sep;35(5):e99. doi: 10.3802/jgo.2024.35.e99. Epub 2024 Apr 22. J Gynecol Oncol. 2024. PMID: 38872480 Free PMC article. Clinical Trial.
-
Bevacizumab combination therapy: a review of its use in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer.BioDrugs. 2013 Aug;27(4):375-92. doi: 10.1007/s40259-013-0043-4. BioDrugs. 2013. PMID: 23728884 Review.
-
Dose-dense approaches to ovarian cancer treatment.Curr Treat Options Oncol. 2015 May;16(5):21. doi: 10.1007/s11864-015-0338-4. Curr Treat Options Oncol. 2015. PMID: 25859831 Review.
Cited by
-
A pilot trial of intravital microscopy in the study of the tumor vasculature of patients with peritoneal carcinomatosis.Sci Rep. 2021 Mar 2;11(1):4946. doi: 10.1038/s41598-021-84430-3. Sci Rep. 2021. PMID: 33654117 Free PMC article.
-
Multiline treatment combining apatinib with toptecan for platinum-resistant recurrent ovarian cancer patients: a report of three cases.Onco Targets Ther. 2018 Apr 6;11:1989-1995. doi: 10.2147/OTT.S158141. eCollection 2018. Onco Targets Ther. 2018. PMID: 29670374 Free PMC article.
-
Continued chemotherapy after complete response to primary therapy among women with advanced ovarian cancer: a meta-analysis.Cancer. 2010 Nov 15;116(22):5251-60. doi: 10.1002/cncr.25487. Cancer. 2010. PMID: 20665885 Free PMC article.
-
Angiogenesis inhibitors for the treatment of epithelial ovarian cancer.Cochrane Database Syst Rev. 2023 Apr 18;4(4):CD007930. doi: 10.1002/14651858.CD007930.pub3. Cochrane Database Syst Rev. 2023. PMID: 37185961 Free PMC article.
-
Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer.Future Oncol. 2020 Mar;16(7):225-246. doi: 10.2217/fon-2019-0042. Epub 2019 Nov 20. Future Oncol. 2020. PMID: 31746224 Free PMC article. Review.
References
-
- United States Food and Drug Administration . United States Food and Drug Administration Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. US Department of Health and Human Services; Rockville, MD: 2007. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati....
-
- Committee for Medicinal Products for Human Use . European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) Guideline for the Evaluation of Anticancer Medicinal Products in Man. London, UK: 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
-
- Amit O, Mannino F, Stone AM, et al. Blinded independent central review of progression in cancer clinical trials: Results from a meta-analysis. Eur J Cancer. 2011;47:1772–8. - PubMed
-
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. New Engl J Med. 2011;365:2473–83. - PubMed
-
- Stuart GC, Kitchener H, Bacon M, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: Report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21:750–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

