The role of mitochondria in cellular iron-sulfur protein biogenesis: mechanisms, connected processes, and diseases
- PMID: 23906713
- PMCID: PMC3721283
- DOI: 10.1101/cshperspect.a011312
The role of mitochondria in cellular iron-sulfur protein biogenesis: mechanisms, connected processes, and diseases
Abstract
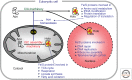
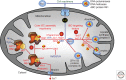
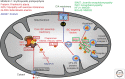
Iron-sulfur (Fe/S) clusters belong to the most ancient protein cofactors in life, and fulfill functions in electron transport, enzyme catalysis, homeostatic regulation, and sulfur activation. The synthesis of Fe/S clusters and their insertion into apoproteins requires almost 30 proteins in the mitochondria and cytosol of eukaryotic cells. This review summarizes our current biochemical knowledge of mitochondrial Fe/S protein maturation. Because this pathway is essential for various extramitochondrial processes, we then explain how mitochondria contribute to the mechanism of cytosolic and nuclear Fe/S protein biogenesis, and to other connected processes including nuclear DNA replication and repair, telomere maintenance, and transcription. We next describe how the efficiency of mitochondria to assemble Fe/S proteins is used to regulate cellular iron homeostasis. Finally, we briefly summarize a number of mitochondrial "Fe/S diseases" in which various biogenesis components are functionally impaired owing to genetic mutations. The thorough understanding of the diverse biochemical disease phenotypes helps with testing the current working model for the molecular mechanism of Fe/S protein biogenesis and its connected processes.
Figures

References
-
- Ajit Bolar N, Vanlander AV, Wilbrecht C, Van der Aa N, Smet J, De Paepe B, Vandeweyer G, Kooy F, Eyskens F, De Latter E, et al. 2013. Mutation of the iron–sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum Mol Genet 22: 2590–2602 - PubMed
-
- Balk J, Pilon M 2011. Ancient and essential: The assembly of iron–sulfur clusters in plants. Trends Plant Sci 16: 218–226 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
