Disruption of the mouse Jhy gene causes abnormal ciliary microtubule patterning and juvenile hydrocephalus
- PMID: 23906841
- PMCID: PMC3783533
- DOI: 10.1016/j.ydbio.2013.07.003
Disruption of the mouse Jhy gene causes abnormal ciliary microtubule patterning and juvenile hydrocephalus
Abstract
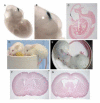
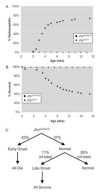
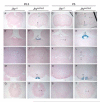
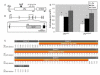
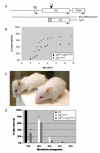
Congenital hydrocephalus, the accumulation of excess cerebrospinal fluid (CSF) in the ventricles of the brain, affects one of every 1000 children born today, making it one of the most common human developmental disorders. Genetic causes of hydrocephalus are poorly understood in humans, but animal models suggest a broad genetic program underlying the regulation of CSF balance. In this study, the random integration of a transgene into the mouse genome led to the development of an early onset and rapidly progressive hydrocephalus. Juvenile hydrocephalus transgenic mice (Jhy(lacZ)) inherit communicating hydrocephalus in an autosomal recessive fashion with dilation of the lateral ventricles observed as early as postnatal day 1.5. Ventricular dilation increases in severity over time, becoming fatal at 4-8 weeks of age. The ependymal cilia lining the lateral ventricles are morphologically abnormal and reduced in number in Jhy(lacZ/lacZ) brains, and ultrastructural analysis revealed disorganization of the expected 9+2 microtubule pattern. Rather, the majority of Jhy(lacZ/lacZ) cilia develop axonemes with 9+0 or 8+2 microtubule structures. Disruption of an unstudied gene, 4931429I11Rik (now named Jhy) appears to underlie the hydrocephalus of Jhy(lacZ/lacZ) mice, and the Jhy transcript and protein are decreased in Jhy(lacZ/lacZ) mice. Partial phenotypic rescue was achieved in Jhy(lacZ/lacZ) mice by the introduction of a bacterial artificial chromosome (BAC) carrying 60-70% of the JHY protein coding sequence. Jhy is evolutionarily conserved from humans to basal vertebrates, but the predicted JHY protein lacks identifiable functional domains. Ongoing studies are directed at uncovering the physiological function of JHY and its role in CSF homeostasis.
Keywords: 4931429I11Rik; Cilia; Hydrocephalus; Jhy; Mouse.
© 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- al-Shroof M, Karnik AM, Karnik AA, Longshore J, Sliman NA, Khan FA. Ciliary dyskinesia associated with hydrocephalus and mental retardation in a Jordanian family. Mayo Clinic Proceedings. 2001;76:1219–1224. - PubMed
-
- Allen ND, Cran DG, Barton SC, Hettle S, Reik W, Surani MA. Transgenes as probes for active chromosomal domains in mouse development. Nature. 1988;333:852–855. - PubMed
-
- Auffray C, Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. European Journal of Biochemistry. 1980;107:303–314. - PubMed
-
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 2009;151C:281–295. - PubMed
-
- Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

