MYC, metabolism, cell growth, and tumorigenesis
- PMID: 23906881
- PMCID: PMC3721271
- DOI: 10.1101/cshperspect.a014217
MYC, metabolism, cell growth, and tumorigenesis
Abstract
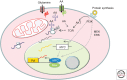
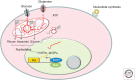
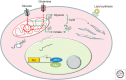
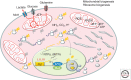
The MYC proto-oncogene is frequently activated in human cancers through a variety of mechanisms. Its deregulated expression, unconstrained by inactivation of key checkpoints, such as p53, contributes to tumorigenesis. Unlike its normal counterpart, which is restrained by negative regulators, the unleashed MYC oncogene produces a transcription factor that alters global gene expression through transcriptional regulation, resulting in tumorigenesis. Key genes involved in ribosomal and mitochondrial biogenesis, glucose and glutamine metabolism, lipid synthesis, and cell-cycle progression are robustly activated by MYC, contributing to the acquisition of bioenergetics substrates for the cancer cell to grow and proliferate.
Figures

References
-
- Askew DS, Ashmun RA, Simmons BC, Cleveland JL 1991. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 6: 1915–1922 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
