NOTCH signaling in Sertoli cells regulates gonocyte fate
- PMID: 23907117
- PMCID: PMC3865042
- DOI: 10.4161/cc.25627
NOTCH signaling in Sertoli cells regulates gonocyte fate
Abstract
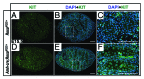
Gonocytes (or prospermatogonia) are the precursors to spermatogonial stem cells (SSCs), which provide the foundation for spermatogenesis through their ability to both self-renew and generate daughter cells. Despite their relative importance, the regulatory mechanisms that govern gonocyte maintenance and transition to SSCs are poorly understood. Recently, we reported that constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence--the first suggestion of the potential role of this signaling pathway in the testis. This Extra View will review what is known about NOTCH signaling, particularly in Sertoli cells and germ cells in the testes, by providing a background on germ cell biology and a summary of our recently published data on NOTCH1 signaling in Sertoli cells. We also describe additional data showing that aberrant proliferation and differentiation of gonocytes in response to constitutive activation of NOTCH1 signaling in Sertoli cells involves de novo expression of cell cycle proteins and a marked upregulation of the KIT receptor. These data further suggest that NOTCH signaling orchestrates a dynamic balance between maintenance and differentiation of gonocytes in the perinatal testis.
Keywords: CCND1; NOTCH signaling; Sertoli cell; gonocyte; mitotic arrest; testis development.
Figures


References
-
- Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
