Fluorescence correlation spectroscopy analysis of serotonin, adrenergic, muscarinic, and dopamine receptor dimerization: the oligomer number puzzle
- PMID: 23907214
- PMCID: PMC3781380
- DOI: 10.1124/mol.113.087072
Fluorescence correlation spectroscopy analysis of serotonin, adrenergic, muscarinic, and dopamine receptor dimerization: the oligomer number puzzle
Abstract
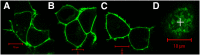
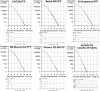
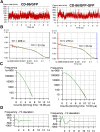
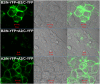
The issue of G protein-coupled receptor (GPCR) oligomer status has not been resolved. Although many studies have provided evidence in favor of receptor-receptor interactions, there is no consensus as to the exact oligomer size of class A GPCRs. Previous studies have reported monomers, dimers, tetramers, and higher-order oligomers. In the present study, this issue was examined using fluorescence correlation spectroscopy (FCS) with photon counting histogram (PCH) analysis, a sensitive method for monitoring diffusion and oligomer size of plasma membrane proteins. Six different class A GPCRs were selected from the serotonin (5-HT2A), adrenergic (α1b-AR and β2-AR), muscarinic (M1 and M2), and dopamine (D1) receptor families. Each GPCR was C-terminally labeled with green fluorescent protein (GFP) or yellow fluorescent protein (YFP) and expressed in human embryonic kidney 293 cells. FCS provided plasma membrane diffusion coefficients on the order of 7.5 × 10(-9) cm(2)/s. PCH molecular brightness analysis was used to determine the GPCR oligomer size. Known monomeric (CD-86) and dimeric (CD-28) receptors with GFP and YFP tags were used as controls to determine the molecular brightness of monomers and dimers. PCH analysis of fluorescence-tagged GPCRs revealed molecular brightness values that were twice the monomeric controls and similar to the dimeric controls. Reduced χ(2) analyses of the PCH data best fit a model for a homogeneous population of homodimers, without tetramers or higher-order oligomers. The homodimer configuration was unaltered by agonist treatment and was stable over a 10-fold range of receptor expression level. The results of this study demonstrate that biogenic amine receptors freely diffusing within the plasma membrane are predominantly homodimers.
Figures

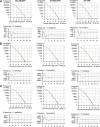

References
-
- Alvarez-Curto E, Ward RJ, Pediani JD, Milligan G. (2010) Ligand regulation of the quaternary organization of cell surface M3 muscarinic acetylcholine receptors analyzed by fluorescence resonance energy transfer (FRET) imaging and homogeneous time-resolved FRET. J Biol Chem 285:23318–23330 - PMC - PubMed
-
- Brea J, Castro M, Giraldo J, López-Giménez JF, Padín JF, Quintián F, Cadavid MI, Vilaró MT, Mengod G, Berg KA, et al. (2009) Evidence for distinct antagonist-revealed functional states of 5-hydroxytryptamine(2A) receptor homodimers. Mol Pharmacol 75:1380–1391 - PubMed
-
- Canals M, Burgueño J, Marcellino D, Cabello N, Canela EI, Mallol J, Agnati L, Ferré S, Bouvier M, Fuxe K, et al. (2004) Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Neurochem 88:726–734 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

