Visual-statistical interpretation of (18)F-FDG-PET images for characteristic Alzheimer patterns in a multicenter study: inter-rater concordance and relationship to automated quantitative evaluation
- PMID: 23907243
- PMCID: PMC7965770
- DOI: 10.3174/ajnr.A3665
Visual-statistical interpretation of (18)F-FDG-PET images for characteristic Alzheimer patterns in a multicenter study: inter-rater concordance and relationship to automated quantitative evaluation
Abstract
Background and purpose: The role of (18)F-FDG-PET in the diagnosis of Alzheimer disease is increasing and should be validated. The aim of this study was to assess the inter-rater variability in the interpretation of (18)F-FDG-PET images obtained in the Japanese Alzheimer's Disease Neuroimaging Initiative, a multicenter clinical research project.
Materials and methods: This study analyzed 274 (18)F-FDG-PET scans (67 mild Alzheimer disease, 100 mild cognitive impairment, and 107 normal cognitive) as baseline scans for the Japanese Alzheimer's Disease Neuroimaging Initiative, which were acquired with various types of PET or PET/CT scanners in 23 facilities. Three independent raters interpreted all PET images by using a combined visual-statistical method. The images were classified into 7 (FDG-7) patterns by the criteria of Silverman et al and further into 2 (FDG-2) patterns.
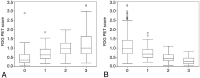
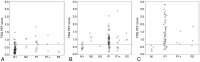
Results: Agreement among the 7 visual-statistical categories by at least 2 of the 3 readers occurred in >94% of cases for all groups: Alzheimer disease, mild cognitive impairment, and normal cognitive. Perfect matches by all 3 raters were observed for 62% of the cases by FDG-7 and 76 by FDG-2. Inter-rater concordance was moderate by FDG-7 (κ = 0.57) and substantial in FDG-2 (κ = 0.67) on average. The FDG-PET score, an automated quantitative index developed by Herholz et al, increased as the number of raters who voted for the AD pattern increased (ρ = 0.59, P < .0001), and the FDG-PET score decreased as those for normal pattern increased (ρ = -0.64, P < .0001).
Conclusions: Inter-rater agreement was moderate to substantial for the combined visual-statistical interpretation of (18)F-FDG-PET and was also significantly associated with automated quantitative assessment.
Figures

References
-
- Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 2001;286:2120–27 - PubMed
-
- Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology 2004;63:2332–40 - PubMed
-
- Yuan Y, Gu ZX, Wei WS. Fluorodeoxyglucose-positron-emission tomography, single-photon emission tomography, and structural MR imaging for prediction of rapid conversion to Alzheimer disease in patients with mild cognitive impairment: a meta-analysis. AJNR Am J Neuroradiol 2009;30:404–10 - PMC - PubMed
-
- Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG-PET. Neuroimage 2002;17:302–16 - PubMed
-
- Herholz K, Westwood S, Haense C, et al. Evaluation of a calibrated 18F-FDG-PET score as a biomarker for progression in Alzheimer disease and mild cognitive impairment. J Nucl Med 2011;52:1218–26 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
