Stromal disrupting effects of nab-paclitaxel in pancreatic cancer
- PMID: 23907428
- PMCID: PMC3749580
- DOI: 10.1038/bjc.2013.415
Stromal disrupting effects of nab-paclitaxel in pancreatic cancer
Abstract
Background: Nab-paclitaxel and gemcitabine have demonstrated a survival benefit over gemcitabine alone in advanced pancreatic cancer (PDA). This study aimed to investigate the clinical, biological, and imaging effects of the regimen in patients with operable PDA.
Methods: Patients with operable PDA received two cycles of nab-paclitaxel and gemcitabine before surgical resection. FDG-PET and CA19.9 tumour marker levels were used to measure clinical activity. Effects on tumour stroma were determined by endoscopic ultrasound (EUS) elastography. The collagen content and architecture as well as density of cancer-associated fibroblasts (CAFs) were determined in the resected surgical specimen and compared with a group of untreated and treated with conventional chemoradiation therapy controls. A co-clinical study in a mouse model of PDA was conducted to differentiate between the effects of nab-paclitaxel and gemcitabine.
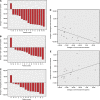
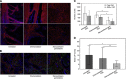
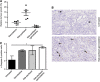
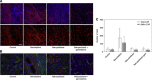
Results: A total of 16 patients were enrolled. Treatment resulted in significant antitumour effects with 50% of patients achieving a >75% decrease in circulating CA19.9 tumour marker and a response by FDG-PET. There was also a significant decrement in tumour stiffness as measured by EUS elastography. Seven of 12 patients who completed treatment and were operated had major pathological regressions. Analysis of residual tumours showed a marked disorganised collagen with a very low density of CAF, which was not observed in the untreated or conventionally treated control groups. The preclinical co-clinical study showed that these effects were specific of nab-paclitaxel and not gemcitabine.
Conclusion: These data suggest that nab-paclitaxel and gemcitabine decreases CAF content inducing a marked alteration in cancer stroma that results in tumour softening. This regimen should be studied in patients with operable PDA.
Figures
Comment in
-
Stellate cells, a point of light in the dark night of pancreatic cancer.Br J Cancer. 2014 Oct 14;111(8):1676-7. doi: 10.1038/bjc.2014.59. Epub 2014 Mar 18. Br J Cancer. 2014. PMID: 24642614 Free PMC article. No abstract available.
-
Neoadjuvant chemotherapy in pancreatic cancer: innovative, but still difficult.Br J Cancer. 2014 Oct 14;111(8):1675-6. doi: 10.1038/bjc.2014.60. Epub 2014 Mar 18. Br J Cancer. 2014. PMID: 24642615 Free PMC article. No abstract available.
-
Reply: 'Comments on Stromal disrupting effects of nab-paclitaxel in pancreatic cancer'.Br J Cancer. 2014 Oct 14;111(8):1677-8. doi: 10.1038/bjc.2014.129. Epub 2014 Mar 18. Br J Cancer. 2014. PMID: 24642623 Free PMC article. No abstract available.
References
-
- Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, Maisonneuve P, Sebastiano PD. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19:1644–1662. - PubMed
-
- Chun YS, Cooper HS, Cohen SJ, Konski A, Burtness B, Denlinger CS, Astsaturov I, Hall MJ, Hoffman JP. Significance of pathologic response to preoperative therapy in pancreatic cancer. Ann Surg Oncol. 2011;18:3601–3607. - PubMed
-
- Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76 (5:953–961. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

